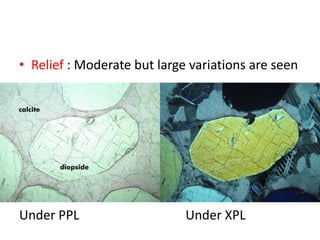
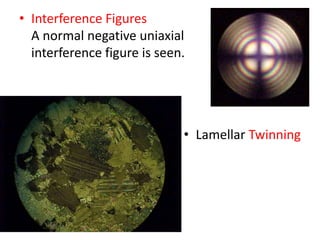
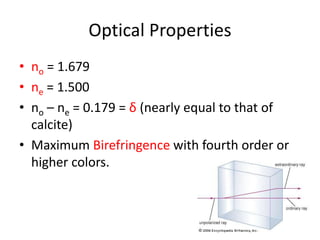
- Calcite and dolomite are common carbonate minerals. Calcite is the most widespread mineral near the Earth's surface, with the chemical formula CaCO3. Dolomite has the formula (CaMg)(CO3)2.
- Calcite is colorless, white, or grey and has a vitreous luster and perfect rhombohedral cleavage. It reacts quickly with acid to produce bubbles of carbon dioxide. Dolomite is also white but contains magnesium. It reacts more slowly with acid and is slightly harder and denser than calcite.
- Both minerals have orthorhombic crystal systems and similar optical properties. Calcite is used as an ornamental stone while