The document discusses crystal structures and their characterization. It covers:
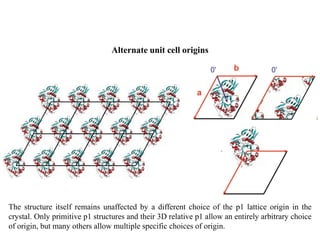
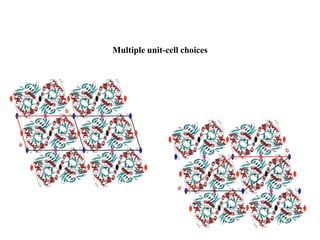
1. Crystals are composed of a repeating motif arranged in a three-dimensional lattice. The motif defines the structural unit and the lattice defines the geometric relationship between motifs.
2. Characterization of crystals includes determining their quality via X-ray diffraction patterns, estimating the number of molecules per unit cell using Matthews coefficient, and deriving the unit cell dimensions and space group from diffraction patterns.
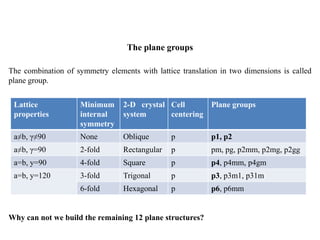
3. Five basic plane lattices exist based on the geometry of the fundamental lattice cell: oblique, rectangular, square, hexagonal, and rectangular I. The combination of lattice symmetry and translation defines the plane crystal system and plane groups.