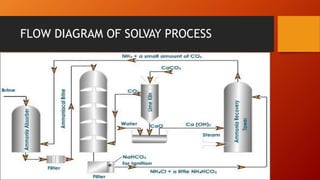
Sodium carbonate, also known as washing soda, has the chemical formula Na2CO3. It occurs naturally as the minerals trona and nahcolite and is mainly produced commercially via the Solvay process. It has important industrial uses in glass, chemicals, detergents, and other products. The Solvay process involves purifying sodium chloride brine, precipitating sodium bicarbonate by reaction of the brine with carbon dioxide and ammonia, and thermally decomposing the bicarbonate to produce sodium carbonate. Trona deposits are also mined and processed to produce sodium carbonate.