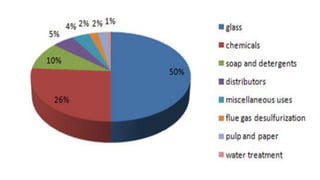
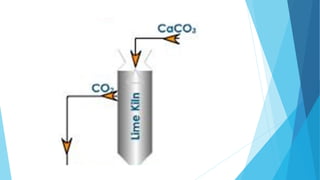
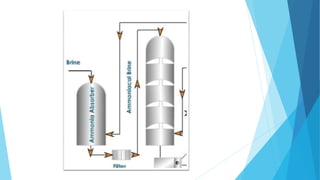
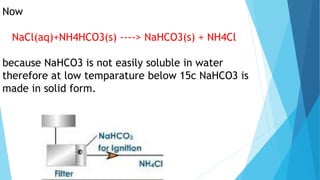
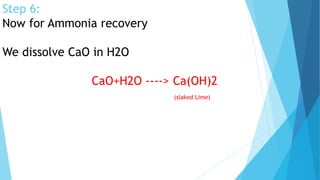
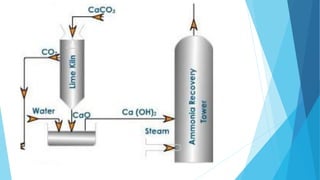
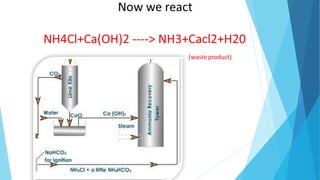
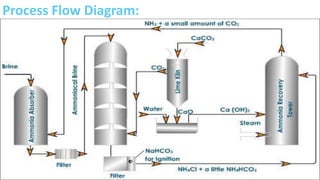
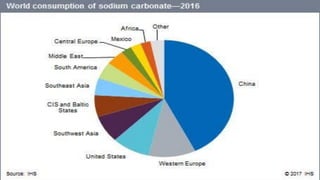
This document summarizes information about sodium carbonate (Na2CO3), including its chemical formula, properties, common names, production methods, and uses. It occurs naturally as a crystalline dehydrate and is highly soluble in water. The most common production methods are the Leblanc process and Solvay process. Sodium carbonate has many industrial and household uses, such as in glassmaking, soap production, water softening, and cleaning products. China is currently the world's largest producer of sodium carbonate.