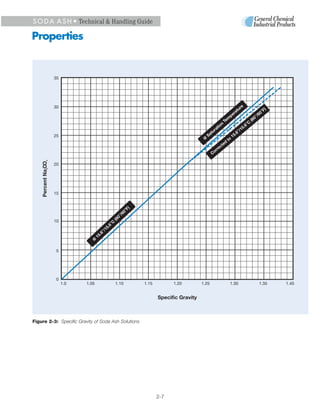
1. Soda ash, also known as sodium carbonate, is produced from trona ore through a purification process. It exists in various solid forms and concentrations in solution.
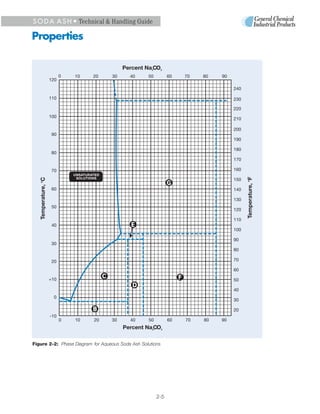
2. Soda ash has maximum solubility of 49.7% at 35.4°C, becoming less soluble at higher temperatures. It hydrates to form different crystalline structures with water.
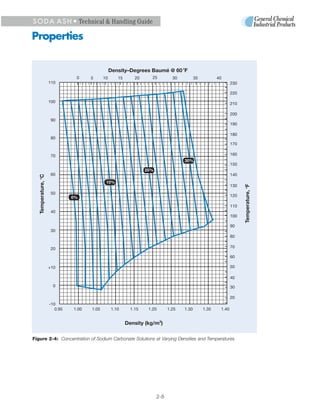
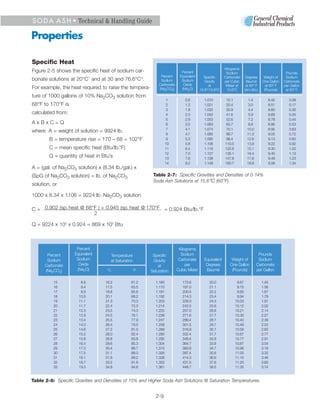
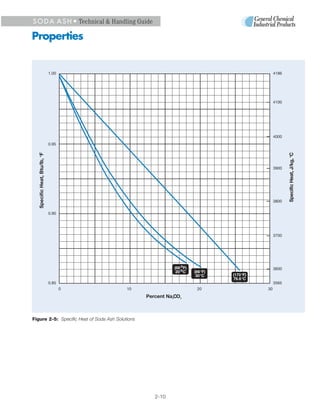
3. Heat is released when soda ash dissolves in water, reaching a maximum of 135 Btu/lb for a 32% saturated solution. Cooling saturated solutions can cause hydrates to precipitate out.




















![S O D A A S H • Technical & Handling Guide
9 Analytical Procedures
CAUTION! 2. Sodium Chloride Reagents
Many of the procedures described below involve •Benzyl alcohol
reagents, indicators and standard solutions that may be •Iron solution indicator
dangerous. These procedures should only be used by •Nitric acid, concentrated
competent analysts who have full knowledge of how to •Potassium thiocyanate, 0.05 N
handle these materials safely. All water used in these •Silver nitrate, 0.05 N
procedures should be distilled or deionized. •Sodium chloride, 0.05 N
The analysis methods below are used at General
Sample Preparation
Chemical’s soda ash facility to monitor soda ash quality.
• For samples having more than 0.1 % NaCl: Weigh
Other methods are available upon request from our
10 g, to the nearest 0.01 g, of a well-mixed sample
Technical Service Department. All reagents listed are
and transfer it to a 300-mL Erlenmeyer flask. Add 25 to
discussed in the last portion of this section.
50 mL of water to dissolve the sample, and carefully
1. Sodium Carbonate Reagents add 15 mL concentrated nitric acid.
•Methyl orange indicator • For samples having less than 0.1 % NaCl: Weigh
•Modified methyl orange indicator 20 g, to the nearest 0.01 g, of a well-mixed sample, and
•Sulfuric acid, 1 N transfer to a 500-mL Erlenmeyer flask. Add 100 mL
Accurately weigh 5.2 ± 0.02 g of a representative water to dissolve the sample, and carefully add 30 mL
sample to the nearest 0.0001 g on an analytical bal- concentrated nitric acid.
ance, and transfer to a 500-mL Erlenmeyer flask. Add
100 mL water, and swirl to dissolve the sample. Add Procedure
5 drops of methyl orange indicator or modified methyl Add 5 mL of iron solution indicator to the prepared
orange indicator solution. Titrate with standard 1 N sample solution. Then add 1.0 mL of 0.05 N potassium
sulfuric acid solution from a 100-mL buret to the pink- thiocyanate solution from a 50-mL Class A buret, and
color end point of methyl orange indicator. If modified swirl to mix. Do not rezero this buret. Using another
methyl orange indicator is used, titrate to a gray end 50-mL Class A buret, titrate with standard 0.05 N silver
point. The same indicator and shade of color as used nitrate solution while vigorously swirling the flask until
for the end point in the standardization of the acid the red color is completely discharged. Then add 2 mL
solution should be used for the sample titration. in excess. Record the volume of silver nitrate solution
Record the volume of titrant used and measure the added. Add 10 drops of benzyl alcohol. Shake the
temperature of the sulfuric acid solution. Correct the flask vigorously for 30 seconds, and rinse down the
volume of titrant to 20°C using Table 9-1. Use the inside wall of the flask with water. Back-titrate with
corrected-volume value for calculation. 0.05 N potassium thiocyanate solution slowly and with
constant swirling until a faint red color persists. (Note 1)
mL H2SO4 at 20°C x N x 5.299 = % Na CO
2 3
Weight of sample [mL AgNO3 - (mL KSCN) (F*)] x N of AgNO3 x 5.84 = % NaCl
Weight of sample
* See last portion of this section for the determination of factor F.
9-1](https://image.slidesharecdn.com/sodaashtech-130416232158-phpapp02/85/Soda-ashtech-39-320.jpg)


![S O D A A S H • Technical & Handling Guide
Analytical Procedures
cell. Using 50-mm cells, measure and record the •Ammonium Hydroxide, Concentrated, 28% NH3.
absorbance of the standard containing no added iron •Ammonium Hydroxide Solution, 1:1 — To 25 ML
and then the absorbances of the remaining standards. of water add 25 mL of reagent grade ammonium
Subtract the absorbance of the zero standard from hydroxide (28-30% NH4OH). Store in a polyethylene
each of the standard absorbances and plot the net dropping bottle.
absorbances against the corresponding micrograms
•Barium Chloride Solution, 100 g/L — Dissolve
of Fe on linear graph paper. Repeat using 10-mm
100 grams of barium chloride (BaCl2·2H2O) in sufficient
absorption cells and the 0 to 400 (g Fe standards
water to make 1000 mL. Filter if turbid.
plotting the net absorbances on a separate sheet of
linear graph paper. •Benzyl Alcohol, C6H5CH2OH — Dispense from an
amber-glass dropping bottle.
Remarks: Before each measurement, check the instrument zero
with the reference cell. Recheck the standard curve at least every •Hydroxylamine Hydrochloride Solution, 100 g/L —
6 months by running a standard of about the same Fe content as
Dissolve 100 g of reagent grade hydroxylamine
the sample along with the sample analysis.
hydrochloride crystals (NH2OH·HCl) in about 600 mL
8. Reagents, Indicators and of water in a 1000-mL beaker. Transfer to a 1000-mL
Standard Solutions volumetric flask, dilute to the mark with water, and mix
Preparation methods for the reagents, indicators and thoroughly. Store in a polyethylene bottle.
standard solutions required by the above procedures •Orthophenanthroline Solution, 3 g/L — Warm
follow. Use reagent grade chemicals, unless otherwise about 500 mL of water in a-1000-mL beaker to
specified, and distilled or deionized water. 60-65°C. Add 3.0 g of reagent grade 1,10-phenanthroline
monohydrate crystals (C12H8N2·H2O) and stir to dissolve.
Reagents
Cool the solution to room temperature. Add 1 mL of
•Acid, Hydrochloric, Concentrated, 36-38% HCl.
reagent grade concentrated hydrochloric acid and mix.
•Acid, Hydrochloric, approximately 10 N — Dilute
Transfer the solution to a 1000-mL volumetric flask,
850 mL of concentrated hydrochloric acid to the mark
dilute to the mark with water and mix well. Store the
in a 1000-mL volumetric flask with water.
solution in a polyethylene bottle.
•Acid, Hydrochloric, Solution, 1:1 — To 25 mL of
•Silver Nitrate Solution, 50 g/L — Dissolve 5 g of
water add 25 mL of reagent grade concentrated
silver nitrate (AgNO3) in water and dilute to 100 mL.
hydrochloric acid (36-38% HCI). Store in a
dropping bottle. Indicators
•Acid, Nitric, Concentrated, 69-71% HNO3. •Iron Solution Indicator — To a 1500-mL beaker
•Acid, Sulfuric, Concentrated, 96-98% H2SO4. add 62 grams of ferric ammonium sulfate
[Fe2(SO4)3·(NH4)2SO4· 24H2O] and 500 mL of water.
•Ammonium Acetate, 50% — Dissolve 500 g of
Stir until the crystals are dissolved. Add 440 mL of con-
reagent grade ammonium acetate crystals (CH3COONH4)
centrated nitric acid and mix. Store the solution in a
in 500 mL of water in a 1500-mL beaker. Mix thoroughly
polyethylene bottle.
and store in a polyethylene bottle.
9-4](https://image.slidesharecdn.com/sodaashtech-130416232158-phpapp02/85/Soda-ashtech-42-320.jpg)
![S O D A A S H • Technical & Handling Guide
Analytical Procedures
•Methyl Orange Indicator — Dissolve 0.1 g of
Normality of Weight of Dried
methyl orange in 100 mL of water. Filter the solution, H2SO4 Solution Na2CO3, Required, g
if necessary.
0.05 0.25-0.26
•Methyl Orange Indicator, Modified — Dissolve 0.10 0.50-0.52
0.1 g of methyl orange and 0.14 g of xylene cyanole 0.20 1.00-1.04
FF dye, technical grade, in 100 mL of water. Filter, 0.50 2.5-2.6
if necessary. 1.0 5.0-5.2
Table 9-1: Normalizing Sulfuric Acid with Dry Soda Ash
Standard Solutions
For precise analytical work, prepare standard solutions
with distilled water at 20°C. Perform all titrations at this Add 100 mL of water to each flask, and swirl to
temperature or apply corrections as given in the tem- dissolve the solid. Add 5 drops of methyl orange indi-
perature correction method given below. cator solution or modified methyl orange indicator
•Acid, Sulfuric, Standard, 0.05-1 N. solution. Titrate with the sulfuric acid solution from a
Measure the required volume* of concentrated sulfuric 100-mL buret to the pink-color end point of methyl
acid in a graduated cylinder, and pour carefully into a orange indicator. If modified methyl orange indicator is
volume of water equal to one half the final desired vol- used, titrate to a gray end point. Record the volume of
ume of solution. Rinse the cylinder into the mixture with sulfuric acid used for each titration. Correct the vol-
water. Mix thoroughly, allow to cool, and dilute to the ume delivered to 20°C as given in the temperature
final desired volume with water. Mix well again and store correction method below. Restandardize monthly.
in a tightly closed glass container. Average the triplicate results.
* The required quantity of concentrated sulfuric acid
g Na2CO3 used x 18.870 = Normality of H2SO4
can be approximated as: mL of H2SO4 corrected to 20°C
Liters of final solution desired mL of concentrated
= sulfuric acid to use. •Iron Solution, Standard, 1 mL = 0.100 mg Fe.
x Normality desired x 30.0
Weigh exactly 0.7022 g of reagent grade ferrous
Transfer 16 g of primary standard grade anhydrous ammonium sulfate hexahydrate [Fe(NH4)2(SO4)2·6H2O],
sodium carbonate to a Pyrex weighing bottle and dry in and transfer it to a 1000-mL volumetric flask containing
an oven at 265 to 285°C for 2 hours or at 250 to about 500 mL of water and 20 mL of concentrated
265°C for 4 hours. Place the cover on the weighing reagent grade sulfuric acid (H2SO4). Swirl to dissolve the
bottle loosely and cool in a desiccator. Weigh, to the crystals, and dilute to the mark with water. Mix thoroughly.
nearest 0.0001 g, three portions of the required weight Discard after one month.
of dried sodium carbonate into three 500-mL Erlen-
•Iron Solution, Working Standard, 1 mL = 10.0
meyer flasks. The weights of dried sodium carbonate
micrograms Fe.
required for the corresponding normalities of sulfuric
To a 1000-mL volumetric flask add about 500 mL
acid solution are shown in Table 9-1.
of water. Pipet exactly 100 mL of the standard Fe
solution (1 mL = 0.100 mg Fe) into the flask. Dilute to
the mark with water and mix thoroughly. Prepare fresh
daily as needed.
9-5](https://image.slidesharecdn.com/sodaashtech-130416232158-phpapp02/85/Soda-ashtech-43-320.jpg)
![S O D A A S H • Technical & Handling Guide
Analytical Procedures
•Potassium Thiocyanate, Standard, 0.05 N. mix. Titrate slowly with the potassium thiocyanate solution, while
constantly swirling, until a faint reddish color persists.
Weigh 4.86 g of potassium thiocyanate (KSCN) to the
nearest 0.01 g in a tared polypropylene weighing bottle. F = mL AgNO3
mL KSCN
Using a wash bottle, quantitatively transfer the crystals Repeat the determination and use the average factor rounded off to
through a powder funnel into a 1-liter volumetric flask. the nearest 0.001 mL AgNO3/mL KSCN. Determine the factor each
time a new KSCN or AgNO3 solution is prepared.
Add 200 to 300 mL of water, and swirl to dissolve the
crystals. Dilute to the mark with water and mix thor- •Sodium Chloride, Standard, 0.05 N.
oughly. Store the solution in a polyethylene bottle. Dry about 3.1 g of sodium chloride (NaCl) on a large
Silver Nitrate, Standard, 0.05N. watch glass in an oven at 105 to 110°C for 2 hours. Cool
Weigh 8.495 g of silver nitrate, (AgNO3) to the nearest the crystals to room temperature in a dessicator. Weigh
0.001 g, in a tared polypropylene weighing bottle. Using 2.9221 g of the dried sodium chloride to the nearest
a water wash bottle, quantitatively transfer the crystals 0.0001 g in a tared polypropylene weighing bottle. Using
through a powder funnel into a 1-liter volumetric flask. a water wash bottle, quantitatively transfer the crystals
Add 200 to 300 mL of water and swirl to dissolve the through a powder funnel to a 1-liter volumetric flask. Add
crystals. Dilute to the mark with water and mix thor- 200 to 300 mL of water and swirl to dissolve the crystals.
oughly. Store in a tightly stoppered amber-glass bottle. Dilute to the mark with water aid mix thoroughly. Store
Standardize the solution as follows. the solution in a polyethylene bottle.
Pipet 25.00 mL of standard 0.05 N sodium chlo-
9. Temperature Corrections
ride solution into each of three 500-mL Erlenmeyer
for Volumetric Solutions
flasks. Carry each flask through the following steps.
As volumetric solutions are standardized at 20°C, titra-
Add 100 mL of water and 3 mL of iron indicator solu-
tions should be made at this temperature or correc-
tion. Swirl to mix. Add 27 mL of the silver nitrate solu-
tions applied to reduce the volume of the solution used
tion being standardized from a 50-mL buret, while
in filtration to 20°C. Table 9-2 shows corrections for
swirling vigorously. Add 10 drops of benzyl alcohol and
temperatures from 15 to 30°C. Columns labelled “mL”
shake the flask for 30 seconds. Rinse down the inside
give the milliliters to be added (≥) or deducted (≤) for
wall of the flask with water. Using a 50-mL buret, back-
each mL used in titration. “Factor” gives the correction
titrate slowly and with constant swirling, with 0.05 N
factor for converting the quantity of solution to stan-
potassium thiocyanate solution until a faint red color
dard volume at 20°C. Corrections for 0.05 N solution
persists. Average the results and restandardize monthly.
apply also to weaker solutions and distilled water.
mL NaCl solution x 0.0500 = Normality of
[mL AgNO3 solution - (mL KSCN solution x F*)] AgNO3
*Determine the factor F as follows: Add about 9 mL of the silver
nitrate solution from the 50-mL buret to a 250-mL Erlenmeyer flask,
Add 100 mL of water and 3 mL of iron indicator solution. Swirl to
9-6](https://image.slidesharecdn.com/sodaashtech-130416232158-phpapp02/85/Soda-ashtech-44-320.jpg)