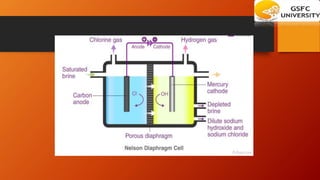
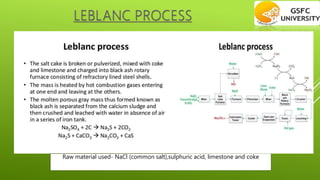
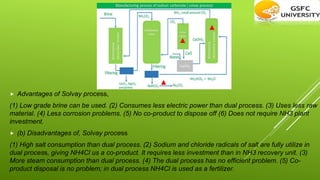
This chapter discusses the manufacture of important industrial chemicals like caustic soda, sodium chloride, soda ash and chlorine gas. It explains the electrolysis process used to produce caustic soda and chlorine as coproducts from salt. Sodium chloride is obtained from salt mines and purified through processes like solar evaporation or vacuum pans. The major industrial process to produce soda ash is the Solvay process which uses common salt, limestone and ammonia as raw materials.