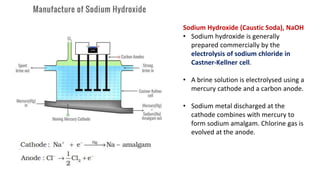
This document discusses important compounds of sodium including sodium carbonate, sodium chloride, and sodium hydroxide. Sodium carbonate is produced via the Solvay process and is used for water softening, laundering, and cleaning. Sodium chloride is obtained from sea water or salt mines and is used as table salt. Sodium hydroxide is produced commercially via electrolysis of sodium chloride and is used to make soap, paper, and chemicals.