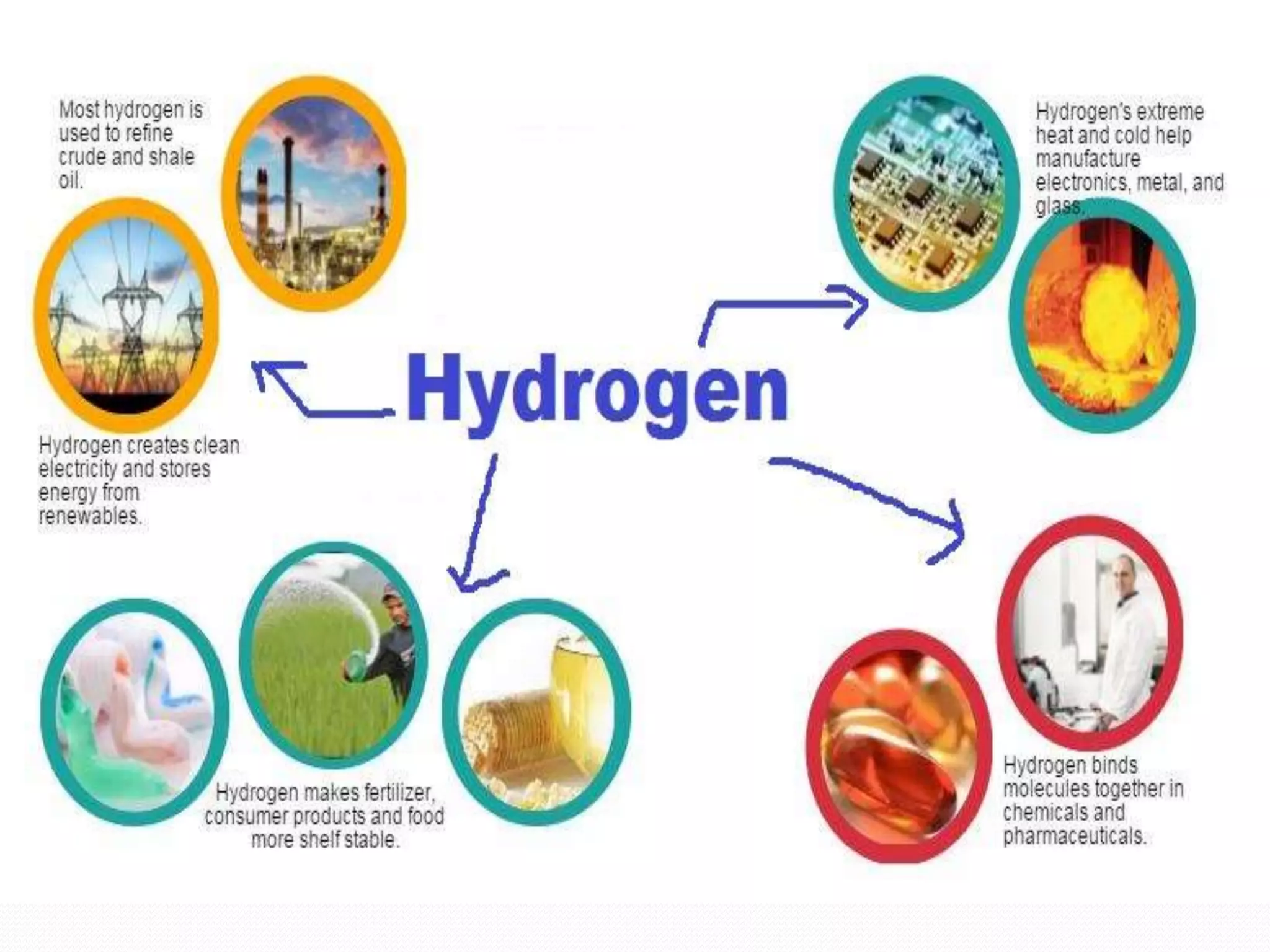
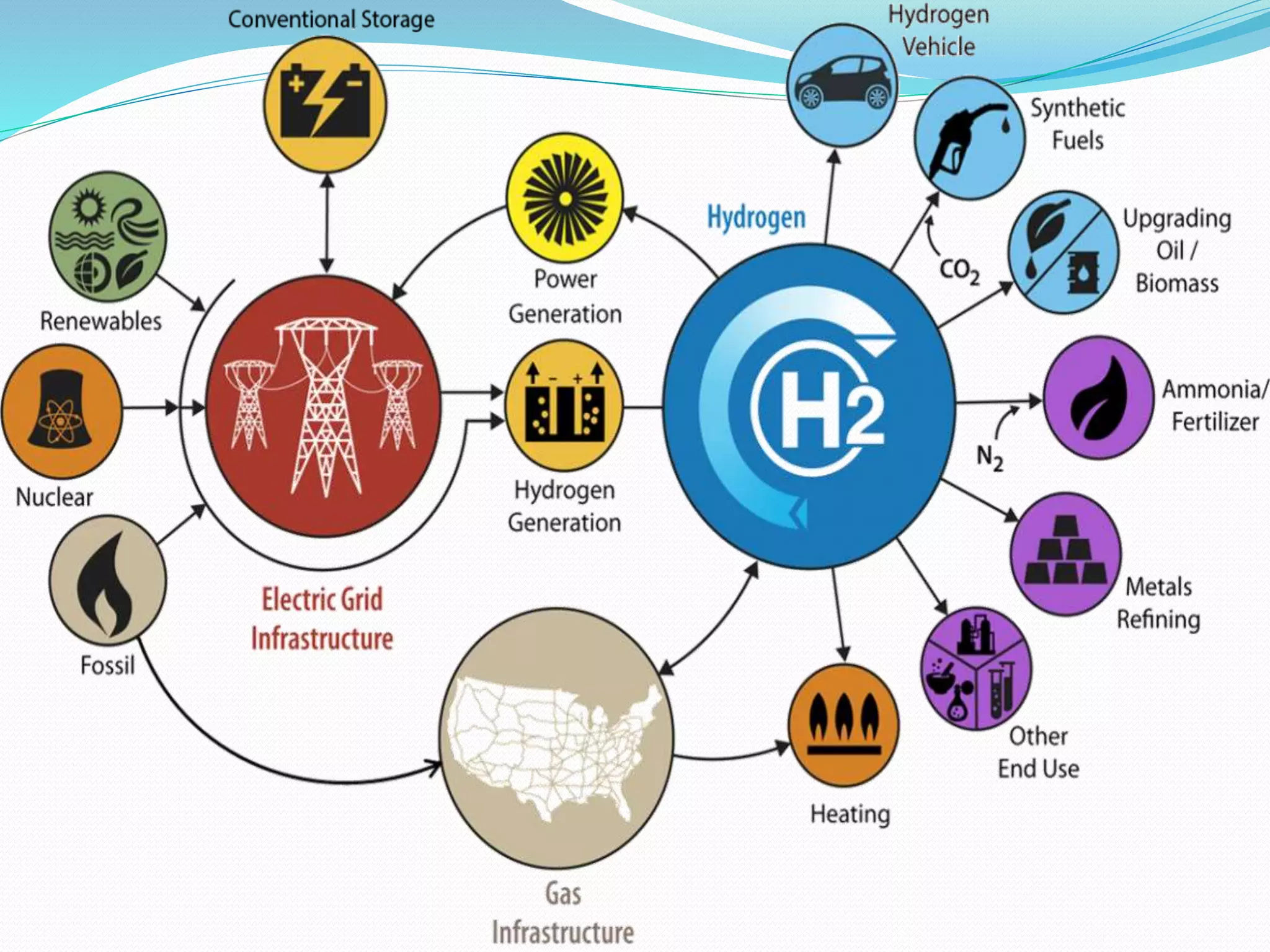
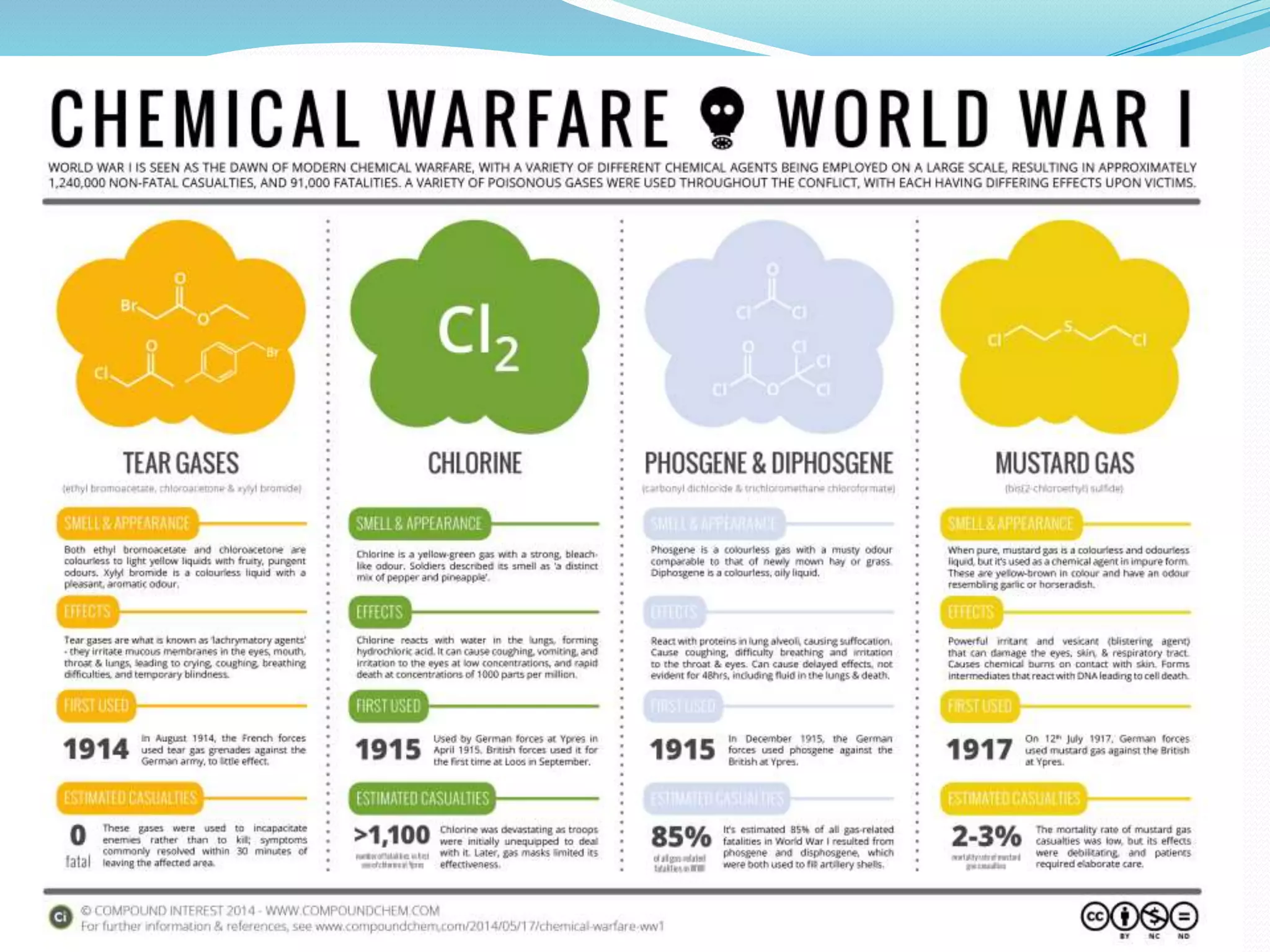
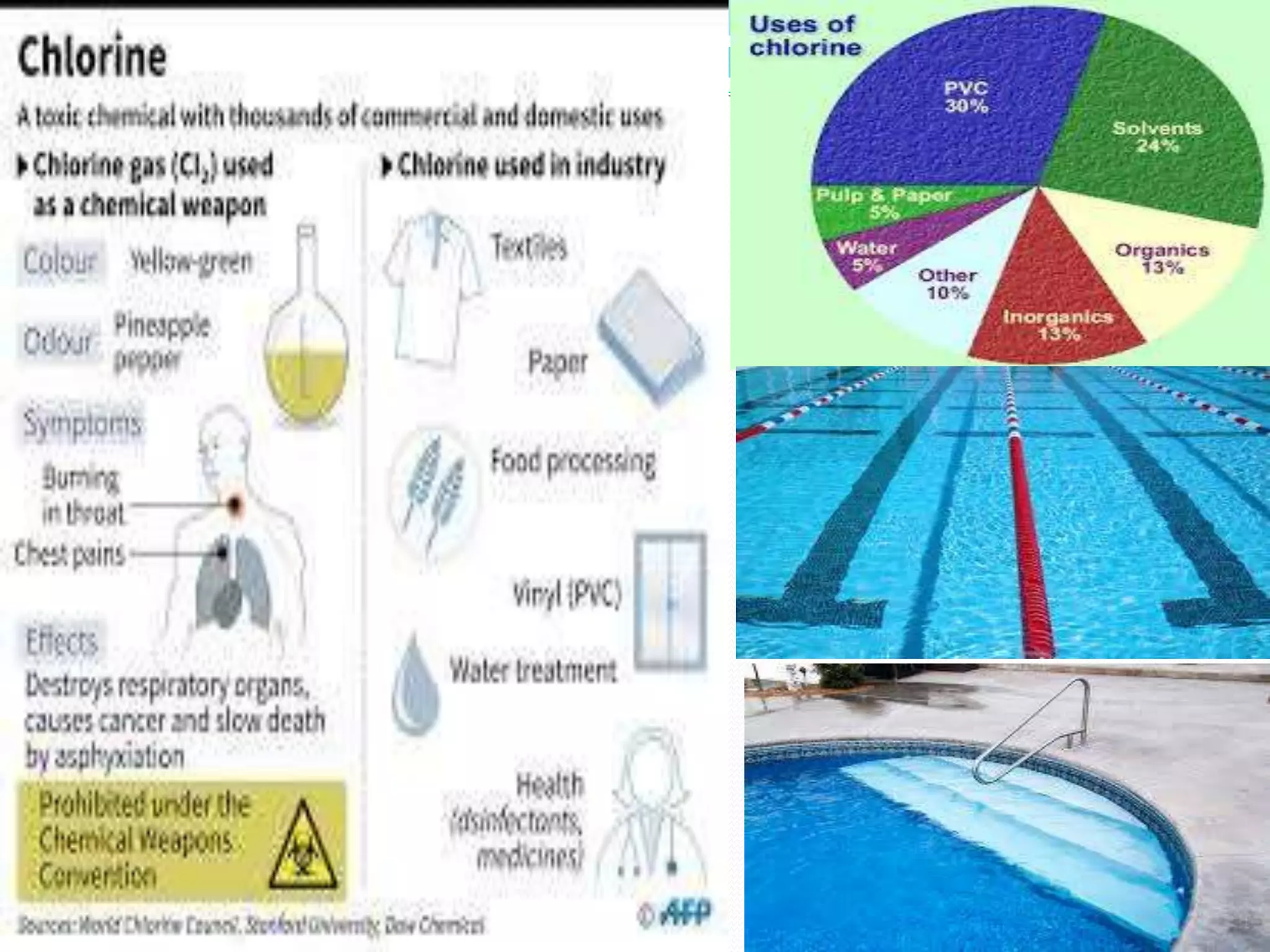
This document discusses several important chemical compounds including common salt, sodium hydroxide, chlorine, hydrogen, baking soda, washing soda, and plaster of Paris. It describes their chemical formulas, methods of production, and key uses. For example, it explains that sodium hydroxide is produced through the chloralkali process of electrolyzing salt water to produce chlorine, hydrogen, and sodium hydroxide. Sodium hydroxide then has various industrial and domestic uses such as for making soap.









![Properties Of Sodium Hydroxides
- Produce hydroxide ions [OH –] in H2O so it is basic in nature.
– It is a water soluble bases so it is called as alkalies.
– It has bitter taste
– It turn red litmus blue.
- It reacts with metals to form oxysalts and release hydrogen
gas
– It act as electrolyte as it produces ions in solution
– it reacts with acids to form salt and water and neutralize
solutions containing H+ ions.
– It has a slippery, ‘soapy’ feel.
– Dissolve fatty material
- It reacts with ammonium salts to release ammonia gas](https://image.slidesharecdn.com/somechemicalcompoundsacidsbasessalts-200529021710/75/Acids-Bases-Salts-Some-Important-chemical-compounds-11-2048.jpg)