s block elements Part5.pptx
•Download as PPTX, PDF•
0 likes•14 views
The document summarizes the general characteristics and properties of compounds of alkali metals such as lithium, sodium, potassium, rubidium, and cesium. It discusses their oxides, hydroxides, halides, oxo-acids, and carbonates. Key points include that the compounds are generally ionic in nature, their physical properties change systematically down the groups (e.g. melting points decrease), and they are generally soluble with high negative enthalpies of formation. Sodium carbonate prepared by the Solvay process is discussed as an important sodium compound used for water softening, laundering, and other industrial applications.
Report
Share
Report
Share
Recommended
Hydrogen (hydrogen, water)

Hydrogen is the first element with one electron. It resembles alkali metals and halogens due to its ability to gain or lose electrons. Hydrogen forms covalent, ionic, and interstitial compounds called hydrides with other elements. Water has many unique properties due to hydrogen bonding between molecules. Hard water contains calcium and magnesium ions that can be removed by boiling, using limewater, or sodium carbonate to produce soft water suitable for household use.
Hydrogen

Hydrogen, the most abundant element in the universe and the third most abundant on the surface of the globe.
All you have to know about this inflammable gas.
10 s-Block Elements.ppt

This document provides information about the s-block elements lithium (Li) through francium (Fr) and the alkaline earth metals beryllium (Be) through radium (Ra). It discusses their electronic configurations, atomic and ionic radii, ionization energies, hydration enthalpies, physical properties, and important compounds such as oxides, hydroxides, halides, and salts. It notes the similarities and differences between lithium and other alkali metals, as well as the similarities between lithium and magnesium. The biological importance of sodium and potassium is also mentioned.
Acids-Bases-Salts @Kidegalize Network_077203774 (1).pdf

This document discusses acids, bases, and salts. It defines acids as compounds that produce hydrogen ions in water, and defines bases as oxides or hydroxides of metals that react with acids to form salts and water. It describes properties of acids such as sour taste and turning litmus red, and properties of bases such as bitter taste and turning litmus blue. The document also discusses the preparation of different types of salts through various reactions, and how their properties depend on whether they are soluble or insoluble in water.
Hydrogen Class 11th Chemistry NCERT 

Hydrogen is the first element in the periodic table. It can form positive ions like alkali metals and negative ions like halogens. It exists as diatomic molecules (H2). There are three isotopes of hydrogen: protium, deuterium, and tritium. Dihydrogen is produced commercially through electrolysis and from the reaction of steam with hydrocarbons. Dihydrogen reacts with many elements and is used to produce ammonia and other important compounds. It forms ionic hydrides with electropositive metals, covalent hydrides with nonmetals, and metallic hydrides with transition metals.
OXIDATIVE REACTION.pptx

This document discusses various oxidative reactions and oxidizing agents. It focuses on non-metallic oxidizing agents such as hydrogen peroxide, sodium hypochlorite, and oxygen gas. For each oxidizing agent, the document describes their industrial production, chemical properties, and common uses. Key oxidative reactions discussed include oxidation of organic compounds, metals, and complexes.
OXIDATION ,PROCESS CHEMISTRY ,MPHARM

OXIDATION ,TYPES OF OXIDATIVE REACTIONS , NON-METALLIC OXIDIZING AGNETS ,LIQUID PHASE OXIDATION, PHARAMACEUTICAL PROCESS CHEMISTRY, MPHARM
Taj

Sodium, magnesium, and aluminium react with oxygen to form ionic oxides. Sodium oxide and magnesium oxide are basic due to their oxide ions and react with water to form alkaline solutions. Aluminium oxide is amphoteric as it displays both acidic and basic properties, reacting with both acids and bases. Silicon dioxide does not react with water or acids due to its covalent bonding. Phosphorus and sulphur form acidic oxides that react with water to produce acids. Chlorine forms oxides that react with water to form acids or salts.
Recommended
Hydrogen (hydrogen, water)

Hydrogen is the first element with one electron. It resembles alkali metals and halogens due to its ability to gain or lose electrons. Hydrogen forms covalent, ionic, and interstitial compounds called hydrides with other elements. Water has many unique properties due to hydrogen bonding between molecules. Hard water contains calcium and magnesium ions that can be removed by boiling, using limewater, or sodium carbonate to produce soft water suitable for household use.
Hydrogen

Hydrogen, the most abundant element in the universe and the third most abundant on the surface of the globe.
All you have to know about this inflammable gas.
10 s-Block Elements.ppt

This document provides information about the s-block elements lithium (Li) through francium (Fr) and the alkaline earth metals beryllium (Be) through radium (Ra). It discusses their electronic configurations, atomic and ionic radii, ionization energies, hydration enthalpies, physical properties, and important compounds such as oxides, hydroxides, halides, and salts. It notes the similarities and differences between lithium and other alkali metals, as well as the similarities between lithium and magnesium. The biological importance of sodium and potassium is also mentioned.
Acids-Bases-Salts @Kidegalize Network_077203774 (1).pdf

This document discusses acids, bases, and salts. It defines acids as compounds that produce hydrogen ions in water, and defines bases as oxides or hydroxides of metals that react with acids to form salts and water. It describes properties of acids such as sour taste and turning litmus red, and properties of bases such as bitter taste and turning litmus blue. The document also discusses the preparation of different types of salts through various reactions, and how their properties depend on whether they are soluble or insoluble in water.
Hydrogen Class 11th Chemistry NCERT 

Hydrogen is the first element in the periodic table. It can form positive ions like alkali metals and negative ions like halogens. It exists as diatomic molecules (H2). There are three isotopes of hydrogen: protium, deuterium, and tritium. Dihydrogen is produced commercially through electrolysis and from the reaction of steam with hydrocarbons. Dihydrogen reacts with many elements and is used to produce ammonia and other important compounds. It forms ionic hydrides with electropositive metals, covalent hydrides with nonmetals, and metallic hydrides with transition metals.
OXIDATIVE REACTION.pptx

This document discusses various oxidative reactions and oxidizing agents. It focuses on non-metallic oxidizing agents such as hydrogen peroxide, sodium hypochlorite, and oxygen gas. For each oxidizing agent, the document describes their industrial production, chemical properties, and common uses. Key oxidative reactions discussed include oxidation of organic compounds, metals, and complexes.
OXIDATION ,PROCESS CHEMISTRY ,MPHARM

OXIDATION ,TYPES OF OXIDATIVE REACTIONS , NON-METALLIC OXIDIZING AGNETS ,LIQUID PHASE OXIDATION, PHARAMACEUTICAL PROCESS CHEMISTRY, MPHARM
Taj

Sodium, magnesium, and aluminium react with oxygen to form ionic oxides. Sodium oxide and magnesium oxide are basic due to their oxide ions and react with water to form alkaline solutions. Aluminium oxide is amphoteric as it displays both acidic and basic properties, reacting with both acids and bases. Silicon dioxide does not react with water or acids due to its covalent bonding. Phosphorus and sulphur form acidic oxides that react with water to produce acids. Chlorine forms oxides that react with water to form acids or salts.
Alkaline earth metals

The document discusses alkaline earth metals, including their extraction methods and properties. It describes:
- Calcium is extracted via electrolysis of molten calcium chloride. Strontium and barium are similarly extracted through electrolysis of their molten chlorides.
- Radium is obtained by electrolysis of molten radium chloride using a mercury cathode and platinum anode.
- Alkaline earth metal salts impart characteristic flame colors from brick red (calcium) to apple green (barium).
- Their reactivity decreases down the group as ionization energy decreases.
Oxidizing agents&ozonolysis

Nonmetallic oxidizing agents such as hydrogen peroxide, sodium hypochlorite, and oxygen gas are discussed. Hydrogen peroxide is an unstable liquid that decomposes into water and oxygen. It is used as a bleaching agent and disinfectant. Sodium hypochlorite is a greenish-yellow solid that decomposes into sodium chloride and chlorine. It is commonly used as a bleach and disinfectant. Oxygen gas makes up 20.8% of the atmosphere and is essential for cellular respiration in living organisms.
Water chemistry using engineering chemistry UNIT-1.pdf

This document summarizes different types of boiler troubles including scales, sludge, and caustic embrittlement formation. It describes how scales and sludge are formed as water becomes more concentrated in a boiler. Scales are hard deposits that form on the inner walls while sludge are softer precipitates. Various internal treatment methods are discussed for removing scales, including carbonate conditioning using sodium carbonate, phosphate conditioning using sodium phosphates, and calgon conditioning. The document also explains demineralization process using ion exchange resins to produce deionized water by exchanging ions from hard water with hydrogen and hydroxide ions. Finally, it defines desalination as removing salts from water and describes electrodialysis as a
Compounds of metals

The document discusses different types of metal compounds including oxides, hydroxides, carbonates, nitrates, and chlorides. It describes methods of preparing these compounds such as direct combination of metals with oxygen or other reactants, or reactions of metal salts with bases or acids. The properties, reactions and uses of these compounds are also outlined. For example, metal oxides can be basic, acidic, or amphoteric and are used to form salts or in manufacturing. Hydroxides vary in solubility depending on the metal's reactivity and react with acids to form salts. Carbonates and nitrates similarly react with acids.
Hydrogen

Hydrogen has many potential industrial applications but faces challenges in production, storage, and safety. It is primarily produced through steam methane reforming, which accounts for 48% of global hydrogen. Other methods include electrolysis and gasification of fossil fuels or biomass. Hydrogen is used in various industrial processes but storage remains an issue due to its low density. Further development is needed to establish hydrogen as a sustainable energy carrier.
12th Chemistry P-block elements Notes for JEE Main 2015 

This document provides information about oxygen and the group 16 elements. It discusses the properties of oxygen and how it differs from other group 16 elements. Some key points include:
- Oxygen exists as a diatomic gas while other group 16 elements form polyatomic solids and liquids.
- Oxygen exhibits different oxidation states and bonding abilities compared to other group 16 elements due to its small size and high electronegativity.
- Common preparation methods for oxygen include thermal decomposition of oxygen-rich salts, metallic oxides, and the reaction of sodium peroxide or potassium permanganate with water.
Carbon and compounds

Diamond has a giant covalent structure where each carbon atom is bonded to four other carbons, giving it properties like hardness and electrical insulation. It is used in cutting, jewelry due to its brilliance, and lasers. Graphite has layers of hexagonal carbon sheets bonded by van der Waals forces, making it soft and a conductor. It is used as a lubricant, in electrodes, and pencils. Hard water contains calcium and magnesium ions that form precipitates with soap. Temporary hardness can be removed by boiling or adding washing soda while permanent hardness requires ion exchange or distillation. Carbon forms oxides, is a reducing agent, and is important in combustion, fermentation, and the carbon cycle.
REDOX TITRATION.pdf

Redox titration, Reduction & Oxidation, Theory & Principle, Redox indicator, Permanganometry, Iodimetry, Iodometry, Dichrometry, Cerimetry, Bromimetry, Titanometry, Titration involving Potassium iodate
Chapter acids, bases and salts(class 10)

This document discusses acids, bases, and salts. It defines acids as substances that produce hydrogen ions (H+) in aqueous solution, making them sour and able to turn litmus red. Bases are defined as substances that produce hydroxide ions (OH-) in solution, making them soapy and able to turn litmus blue. Salts are formed by the reaction of acids and bases and can be acidic, basic, or neutral depending on the reactants. Common natural and synthetic acid-base indicators are also described. The document then discusses the properties and reactions of acids, bases, and salts and how pH is used to measure acidity. Finally, several industrial chemicals derived from sodium chloride (common salt) are summarized, including
Selected synthetic methods in pharmaceutical chemistry.pptx

Selected synthetic methods in Pharmaceutical Biotechnology.
Types of Salts and solubility rate

CHEMISTRY : ACIDS,BASES AND SALTS:
1.definition and characteristics of salts,
2. preparation of salts and,
3. types of salts,
4.uses and
5.hydrolysis of salt in water
Bases

Bases are substances that can accept protons and release hydroxide ions in water. They have properties such as turning litmus paper blue, reacting with acids to form salts, and having a pH greater than 7 when dissolved in water. Common bases include alkali metal and alkaline earth metal hydroxides like NaOH and Ca(OH)2. Bases are classified based on their strength as strong, weak, or superbases. They can also be classified as organic or inorganic based on their source.
Chemical Reaction & Equation class 10.pptx

This document discusses chemical reactions and equations. It defines a chemical reaction as the transformation of one or more substances into new substances through bond breaking and forming. Reactants are the original substances, while products are the new substances formed. Chemical equations represent reactions symbolically, with balanced equations showing equal numbers of each type of atom on both sides. The document describes several types of chemical reactions including combination, decomposition, displacement, double displacement, and oxidation-reduction reactions. It provides examples to illustrate characteristic properties of reactions like gas evolution, precipitation, temperature change, and state changes. Oxidation and reduction reactions are explained along with examples of their effects in corrosion and food rancidity.
acid base.pptx

This document discusses the chemical properties of acids and bases. It states that acids react with metals to produce hydrogen gas, with metal oxides to produce salts and water, and with metal carbonates and bicarbonates to produce salts, water and carbon dioxide gas. Acids also react with bases in a neutralization reaction to produce salts and water. It distinguishes between strong acids that fully ionize in water and weak acids that partially ionize. For bases, it notes they react with metals to produce hydrogen gas, with non-metallic oxides to produce salts and water, and with acids in a neutralization reaction. It also defines strong bases as those that fully release hydroxyl ions in water and weak bases as those
s block elements part 6.pptx

This document discusses important compounds of sodium including sodium carbonate, sodium chloride, and sodium hydroxide. Sodium carbonate is produced via the Solvay process and is used for water softening, laundering, and cleaning. Sodium chloride is obtained from sea water or salt mines and is used as table salt. Sodium hydroxide is produced commercially via electrolysis of sodium chloride and is used to make soap, paper, and chemicals.
C1 revision powerpoint

The early atmosphere on Earth was formed by gases released from volcanic eruptions. The main gases were carbon dioxide, nitrogen, water vapor, and ammonia, with little to no oxygen. Over time, carbon dioxide levels fell as it dissolved in the oceans and was incorporated into marine organisms' shells. As plant life increased through photosynthesis, oxygen levels rose and carbon dioxide levels fell further. Rocks can provide information about the early atmosphere by analyzing their mineral composition and looking for oxide formations that indicate higher oxygen levels over time.
Alkali metals Grade 11 Chemistry

The document discusses the properties and extraction of sodium and its compounds. It describes that sodium is extracted through electrolysis of molten sodium chloride using the Downs process. Sodium reacts violently with water and acids, forming sodium hydroxide and hydrogen gas. Sodium hydroxide and sodium carbonate are important industrial compounds used to make paper, soap and glass.
Acids, bases and salt

This document discusses acids, bases, and salts. It defines acids as substances that produce hydrogen ions in solution and turn litmus blue. Bases are defined as substances that produce hydroxide ions in solution and turn litmus red. Strong acids fully ionize in solution, while weak acids only partially ionize. Salts are formed from the reaction of acids and bases. Properties of acids, bases, and salts are discussed such as taste, effect on indicators, and solubility. Methods of preparing different types of salts are also outlined.
Acid Base And Salt.pdf

1) Acids are compounds that form hydrogen (H+) ions in solution and have a pH less than 7. Common strong acids include hydrochloric acid, nitric acid, and sulfuric acid.
2) Bases are compounds that form hydroxide (OH-) ions in solution and have a pH greater than 7. Common bases include sodium hydroxide, calcium hydroxide, and ammonium hydroxide.
3) A salt is formed through a neutralization reaction between an acid and a base, producing water and an ionic compound composed of the cation from the base and the anion from the acid. Examples of salts include sodium chloride, magnesium sulfate, and ammonium nitrate.
Acids, bases and salts according to the syllabus of CAIE and IGCSE

This document provides information about acids and bases:
- Acids are substances that produce hydrogen ions in water and have properties like a sour taste and turning litmus paper red. Strong acids fully ionize in water while weak acids only partially ionize.
- Bases include metal oxides and hydroxides. Soluble bases are called alkalis. Bases react with acids to produce salt and water in a neutralization reaction.
- Important acids and bases have many applications from batteries to cleaning to food preservation. Processes like the Contact Process are used industrially to produce acids like sulfuric acid.
s block elements Part4.pptx

The alkali metals are highly reactive due to their large size and low ionization enthalpy. Their reactivity increases down the group. They react vigorously with water to form hydroxides and dihydrogen gas. Lithium reacts less vigorously than the other alkali metals due to its smaller size and high hydration energy. The other alkali metals react explosively with water and also react with proton donors like alcohols, ammonia, and alkynes.
s block elements Part 3.pptx

The document discusses several properties of alkali metals. It states that the hydration enthalpy of ions, which is the heat released when ions dissolve in water, is always negative and decreases with increasing ionic size from Li+ to Cs+. It also notes that density increases down the group, though K is lighter than Na. Different alkali metals and their salts impart different colors to flames due to electron excitation and emission. Detection methods include flame tests, flame photometry, and atomic absorption spectroscopy. Chemical reactivity increases down the group as ionization enthalpy decreases.
More Related Content
Similar to s block elements Part5.pptx
Alkaline earth metals

The document discusses alkaline earth metals, including their extraction methods and properties. It describes:
- Calcium is extracted via electrolysis of molten calcium chloride. Strontium and barium are similarly extracted through electrolysis of their molten chlorides.
- Radium is obtained by electrolysis of molten radium chloride using a mercury cathode and platinum anode.
- Alkaline earth metal salts impart characteristic flame colors from brick red (calcium) to apple green (barium).
- Their reactivity decreases down the group as ionization energy decreases.
Oxidizing agents&ozonolysis

Nonmetallic oxidizing agents such as hydrogen peroxide, sodium hypochlorite, and oxygen gas are discussed. Hydrogen peroxide is an unstable liquid that decomposes into water and oxygen. It is used as a bleaching agent and disinfectant. Sodium hypochlorite is a greenish-yellow solid that decomposes into sodium chloride and chlorine. It is commonly used as a bleach and disinfectant. Oxygen gas makes up 20.8% of the atmosphere and is essential for cellular respiration in living organisms.
Water chemistry using engineering chemistry UNIT-1.pdf

This document summarizes different types of boiler troubles including scales, sludge, and caustic embrittlement formation. It describes how scales and sludge are formed as water becomes more concentrated in a boiler. Scales are hard deposits that form on the inner walls while sludge are softer precipitates. Various internal treatment methods are discussed for removing scales, including carbonate conditioning using sodium carbonate, phosphate conditioning using sodium phosphates, and calgon conditioning. The document also explains demineralization process using ion exchange resins to produce deionized water by exchanging ions from hard water with hydrogen and hydroxide ions. Finally, it defines desalination as removing salts from water and describes electrodialysis as a
Compounds of metals

The document discusses different types of metal compounds including oxides, hydroxides, carbonates, nitrates, and chlorides. It describes methods of preparing these compounds such as direct combination of metals with oxygen or other reactants, or reactions of metal salts with bases or acids. The properties, reactions and uses of these compounds are also outlined. For example, metal oxides can be basic, acidic, or amphoteric and are used to form salts or in manufacturing. Hydroxides vary in solubility depending on the metal's reactivity and react with acids to form salts. Carbonates and nitrates similarly react with acids.
Hydrogen

Hydrogen has many potential industrial applications but faces challenges in production, storage, and safety. It is primarily produced through steam methane reforming, which accounts for 48% of global hydrogen. Other methods include electrolysis and gasification of fossil fuels or biomass. Hydrogen is used in various industrial processes but storage remains an issue due to its low density. Further development is needed to establish hydrogen as a sustainable energy carrier.
12th Chemistry P-block elements Notes for JEE Main 2015 

This document provides information about oxygen and the group 16 elements. It discusses the properties of oxygen and how it differs from other group 16 elements. Some key points include:
- Oxygen exists as a diatomic gas while other group 16 elements form polyatomic solids and liquids.
- Oxygen exhibits different oxidation states and bonding abilities compared to other group 16 elements due to its small size and high electronegativity.
- Common preparation methods for oxygen include thermal decomposition of oxygen-rich salts, metallic oxides, and the reaction of sodium peroxide or potassium permanganate with water.
Carbon and compounds

Diamond has a giant covalent structure where each carbon atom is bonded to four other carbons, giving it properties like hardness and electrical insulation. It is used in cutting, jewelry due to its brilliance, and lasers. Graphite has layers of hexagonal carbon sheets bonded by van der Waals forces, making it soft and a conductor. It is used as a lubricant, in electrodes, and pencils. Hard water contains calcium and magnesium ions that form precipitates with soap. Temporary hardness can be removed by boiling or adding washing soda while permanent hardness requires ion exchange or distillation. Carbon forms oxides, is a reducing agent, and is important in combustion, fermentation, and the carbon cycle.
REDOX TITRATION.pdf

Redox titration, Reduction & Oxidation, Theory & Principle, Redox indicator, Permanganometry, Iodimetry, Iodometry, Dichrometry, Cerimetry, Bromimetry, Titanometry, Titration involving Potassium iodate
Chapter acids, bases and salts(class 10)

This document discusses acids, bases, and salts. It defines acids as substances that produce hydrogen ions (H+) in aqueous solution, making them sour and able to turn litmus red. Bases are defined as substances that produce hydroxide ions (OH-) in solution, making them soapy and able to turn litmus blue. Salts are formed by the reaction of acids and bases and can be acidic, basic, or neutral depending on the reactants. Common natural and synthetic acid-base indicators are also described. The document then discusses the properties and reactions of acids, bases, and salts and how pH is used to measure acidity. Finally, several industrial chemicals derived from sodium chloride (common salt) are summarized, including
Selected synthetic methods in pharmaceutical chemistry.pptx

Selected synthetic methods in Pharmaceutical Biotechnology.
Types of Salts and solubility rate

CHEMISTRY : ACIDS,BASES AND SALTS:
1.definition and characteristics of salts,
2. preparation of salts and,
3. types of salts,
4.uses and
5.hydrolysis of salt in water
Bases

Bases are substances that can accept protons and release hydroxide ions in water. They have properties such as turning litmus paper blue, reacting with acids to form salts, and having a pH greater than 7 when dissolved in water. Common bases include alkali metal and alkaline earth metal hydroxides like NaOH and Ca(OH)2. Bases are classified based on their strength as strong, weak, or superbases. They can also be classified as organic or inorganic based on their source.
Chemical Reaction & Equation class 10.pptx

This document discusses chemical reactions and equations. It defines a chemical reaction as the transformation of one or more substances into new substances through bond breaking and forming. Reactants are the original substances, while products are the new substances formed. Chemical equations represent reactions symbolically, with balanced equations showing equal numbers of each type of atom on both sides. The document describes several types of chemical reactions including combination, decomposition, displacement, double displacement, and oxidation-reduction reactions. It provides examples to illustrate characteristic properties of reactions like gas evolution, precipitation, temperature change, and state changes. Oxidation and reduction reactions are explained along with examples of their effects in corrosion and food rancidity.
acid base.pptx

This document discusses the chemical properties of acids and bases. It states that acids react with metals to produce hydrogen gas, with metal oxides to produce salts and water, and with metal carbonates and bicarbonates to produce salts, water and carbon dioxide gas. Acids also react with bases in a neutralization reaction to produce salts and water. It distinguishes between strong acids that fully ionize in water and weak acids that partially ionize. For bases, it notes they react with metals to produce hydrogen gas, with non-metallic oxides to produce salts and water, and with acids in a neutralization reaction. It also defines strong bases as those that fully release hydroxyl ions in water and weak bases as those
s block elements part 6.pptx

This document discusses important compounds of sodium including sodium carbonate, sodium chloride, and sodium hydroxide. Sodium carbonate is produced via the Solvay process and is used for water softening, laundering, and cleaning. Sodium chloride is obtained from sea water or salt mines and is used as table salt. Sodium hydroxide is produced commercially via electrolysis of sodium chloride and is used to make soap, paper, and chemicals.
C1 revision powerpoint

The early atmosphere on Earth was formed by gases released from volcanic eruptions. The main gases were carbon dioxide, nitrogen, water vapor, and ammonia, with little to no oxygen. Over time, carbon dioxide levels fell as it dissolved in the oceans and was incorporated into marine organisms' shells. As plant life increased through photosynthesis, oxygen levels rose and carbon dioxide levels fell further. Rocks can provide information about the early atmosphere by analyzing their mineral composition and looking for oxide formations that indicate higher oxygen levels over time.
Alkali metals Grade 11 Chemistry

The document discusses the properties and extraction of sodium and its compounds. It describes that sodium is extracted through electrolysis of molten sodium chloride using the Downs process. Sodium reacts violently with water and acids, forming sodium hydroxide and hydrogen gas. Sodium hydroxide and sodium carbonate are important industrial compounds used to make paper, soap and glass.
Acids, bases and salt

This document discusses acids, bases, and salts. It defines acids as substances that produce hydrogen ions in solution and turn litmus blue. Bases are defined as substances that produce hydroxide ions in solution and turn litmus red. Strong acids fully ionize in solution, while weak acids only partially ionize. Salts are formed from the reaction of acids and bases. Properties of acids, bases, and salts are discussed such as taste, effect on indicators, and solubility. Methods of preparing different types of salts are also outlined.
Acid Base And Salt.pdf

1) Acids are compounds that form hydrogen (H+) ions in solution and have a pH less than 7. Common strong acids include hydrochloric acid, nitric acid, and sulfuric acid.
2) Bases are compounds that form hydroxide (OH-) ions in solution and have a pH greater than 7. Common bases include sodium hydroxide, calcium hydroxide, and ammonium hydroxide.
3) A salt is formed through a neutralization reaction between an acid and a base, producing water and an ionic compound composed of the cation from the base and the anion from the acid. Examples of salts include sodium chloride, magnesium sulfate, and ammonium nitrate.
Acids, bases and salts according to the syllabus of CAIE and IGCSE

This document provides information about acids and bases:
- Acids are substances that produce hydrogen ions in water and have properties like a sour taste and turning litmus paper red. Strong acids fully ionize in water while weak acids only partially ionize.
- Bases include metal oxides and hydroxides. Soluble bases are called alkalis. Bases react with acids to produce salt and water in a neutralization reaction.
- Important acids and bases have many applications from batteries to cleaning to food preservation. Processes like the Contact Process are used industrially to produce acids like sulfuric acid.
Similar to s block elements Part5.pptx (20)
Water chemistry using engineering chemistry UNIT-1.pdf

Water chemistry using engineering chemistry UNIT-1.pdf
12th Chemistry P-block elements Notes for JEE Main 2015 

12th Chemistry P-block elements Notes for JEE Main 2015
Selected synthetic methods in pharmaceutical chemistry.pptx

Selected synthetic methods in pharmaceutical chemistry.pptx
Acids, bases and salts according to the syllabus of CAIE and IGCSE

Acids, bases and salts according to the syllabus of CAIE and IGCSE
More from DishaAnkar
s block elements Part4.pptx

The alkali metals are highly reactive due to their large size and low ionization enthalpy. Their reactivity increases down the group. They react vigorously with water to form hydroxides and dihydrogen gas. Lithium reacts less vigorously than the other alkali metals due to its smaller size and high hydration energy. The other alkali metals react explosively with water and also react with proton donors like alcohols, ammonia, and alkynes.
s block elements Part 3.pptx

The document discusses several properties of alkali metals. It states that the hydration enthalpy of ions, which is the heat released when ions dissolve in water, is always negative and decreases with increasing ionic size from Li+ to Cs+. It also notes that density increases down the group, though K is lighter than Na. Different alkali metals and their salts impart different colors to flames due to electron excitation and emission. Detection methods include flame tests, flame photometry, and atomic absorption spectroscopy. Chemical reactivity increases down the group as ionization enthalpy decreases.
s block elements part 2.pptx

The alkali metals have low ionization enthalpies that decrease down the group from lithium to cesium. This is because the increasing atomic size outweighs the effect of the increasing nuclear charge, so the outermost electron is well shielded from the positive charge of the nucleus.
s block elements.pptx

The s-block elements are those where the last electron enters the s-orbital. Groups 1 and 2 belong to the s-block. Group 1 elements are alkali metals including lithium, sodium, potassium, rubidium, cesium, and francium. Group 2 elements are alkaline earth metals including beryllium, magnesium, calcium, strontium, barium, and radium. The alkali metals have one valence electron and readily lose electrons to form M+ ions, so they are never found in free state in nature.
CARBOHYDRATE.pptx

Carbohydrates are polyhydroxy aldehydes or ketones that are primarily produced by plants. They can be classified as monosaccharides, disaccharides, oligosaccharides, or polysaccharides depending on their structure. Monosaccharides are the simplest form and include both aldoses and ketoses containing an aldehyde or ketone functional group. Disaccharides yield two monosaccharide units upon hydrolysis, such as sucrose hydrolyzing to glucose and fructose. Polysaccharides are carbohydrates that yield many monosaccharide units when hydrolyzed and include non-sugars like starch and cellulose. Carbohydrates undergo mutarotation where their optical rotation changes as they reach
LSTM based method oh ML.pptx

This document provides an introduction to a seminar on using deep learning and LSTM methods for stock price prediction. It discusses using machine learning algorithms to analyze stock market data and predict future prices. The proposed system would use tools like Pandas, NumPy, and Scikit-learn to get stock data and predict prices using an LSTM model. It presents the modules, architecture, advantages, and requirements of the system. The conclusion states that both machine learning techniques showed improved prediction accuracy compared to traditional methods, with LSTM proving more efficient.
flowchart ON DEEP LEARNING SPP

This document provides an introductory seminar on using deep learning and LSTM methods for stock price prediction. It discusses using machine learning to analyze stock market data and predict future prices. The objectives are to accurately predict stock prices and provide a mobile app interface for visualization. Literature on existing prediction systems is reviewed and a proposed system is described using tools like Pandas, NumPy, and Scikit-learn. The system architecture involves data processing, dimension reduction, model training with LSTM, and price prediction. Potential advantages include automation and continuous improvement, while disadvantages include time/resources and error-prone models.
stock prise prediction.pptx

This document provides an introductory seminar presentation on using deep learning and LSTM methods for stock price prediction. The presentation was given by students at the Manoharbhai Patel Institute of Engineering & Technology under the guidance of Prof. OMKAR DUDBHURE. The presentation covers the objectives of stock price prediction using machine learning techniques, a literature review on existing approaches, a description of the proposed system including modules and tools, an overview of the system architecture and flowchart, advantages and disadvantages, and references. The goal is to accurately predict future stock prices using past stock market data and deep learning models like LSTM to help investors.
haemoglobin by disha ankar 

Porphyrins serve as prosthetic groups for proteins involved in oxygen transport and other functions. Hemoglobin contains heme, an iron-containing porphyrin, at the center of each subunit. Heme synthesis involves several steps beginning with the condensation of glycine and succinyl-CoA to form 5-aminolevulinate. Regulation occurs through feedback inhibition by heme. Hemoglobin transports oxygen through a sigmoidal binding curve conferred by cooperativity between subunits. Pathologies can result from oxidation, genetic mutations like sickle cell anemia, or defects in subunit synthesis as seen in thalassemias.
More from DishaAnkar (9)
Recently uploaded
Deep Software Variability and Frictionless Reproducibility

Deep Software Variability and Frictionless ReproducibilityUniversity of Rennes, INSA Rennes, Inria/IRISA, CNRS
The ability to recreate computational results with minimal effort and actionable metrics provides a solid foundation for scientific research and software development. When people can replicate an analysis at the touch of a button using open-source software, open data, and methods to assess and compare proposals, it significantly eases verification of results, engagement with a diverse range of contributors, and progress. However, we have yet to fully achieve this; there are still many sociotechnical frictions.
Inspired by David Donoho's vision, this talk aims to revisit the three crucial pillars of frictionless reproducibility (data sharing, code sharing, and competitive challenges) with the perspective of deep software variability.
Our observation is that multiple layers — hardware, operating systems, third-party libraries, software versions, input data, compile-time options, and parameters — are subject to variability that exacerbates frictions but is also essential for achieving robust, generalizable results and fostering innovation. I will first review the literature, providing evidence of how the complex variability interactions across these layers affect qualitative and quantitative software properties, thereby complicating the reproduction and replication of scientific studies in various fields.
I will then present some software engineering and AI techniques that can support the strategic exploration of variability spaces. These include the use of abstractions and models (e.g., feature models), sampling strategies (e.g., uniform, random), cost-effective measurements (e.g., incremental build of software configurations), and dimensionality reduction methods (e.g., transfer learning, feature selection, software debloating).
I will finally argue that deep variability is both the problem and solution of frictionless reproducibility, calling the software science community to develop new methods and tools to manage variability and foster reproducibility in software systems.
Exposé invité Journées Nationales du GDR GPL 2024
The use of Nauplii and metanauplii artemia in aquaculture (brine shrimp).pptx

Although Artemia has been known to man for centuries, its use as a food for the culture of larval organisms apparently began only in the 1930s, when several investigators found that it made an excellent food for newly hatched fish larvae (Litvinenko et al., 2023). As aquaculture developed in the 1960s and ‘70s, the use of Artemia also became more widespread, due both to its convenience and to its nutritional value for larval organisms (Arenas-Pardo et al., 2024). The fact that Artemia dormant cysts can be stored for long periods in cans, and then used as an off-the-shelf food requiring only 24 h of incubation makes them the most convenient, least labor-intensive, live food available for aquaculture (Sorgeloos & Roubach, 2021). The nutritional value of Artemia, especially for marine organisms, is not constant, but varies both geographically and temporally. During the last decade, however, both the causes of Artemia nutritional variability and methods to improve poorquality Artemia have been identified (Loufi et al., 2024).
Brine shrimp (Artemia spp.) are used in marine aquaculture worldwide. Annually, more than 2,000 metric tons of dry cysts are used for cultivation of fish, crustacean, and shellfish larva. Brine shrimp are important to aquaculture because newly hatched brine shrimp nauplii (larvae) provide a food source for many fish fry (Mozanzadeh et al., 2021). Culture and harvesting of brine shrimp eggs represents another aspect of the aquaculture industry. Nauplii and metanauplii of Artemia, commonly known as brine shrimp, play a crucial role in aquaculture due to their nutritional value and suitability as live feed for many aquatic species, particularly in larval stages (Sorgeloos & Roubach, 2021).
The binding of cosmological structures by massless topological defects

Assuming spherical symmetry and weak field, it is shown that if one solves the Poisson equation or the Einstein field
equations sourced by a topological defect, i.e. a singularity of a very specific form, the result is a localized gravitational
field capable of driving flat rotation (i.e. Keplerian circular orbits at a constant speed for all radii) of test masses on a thin
spherical shell without any underlying mass. Moreover, a large-scale structure which exploits this solution by assembling
concentrically a number of such topological defects can establish a flat stellar or galactic rotation curve, and can also deflect
light in the same manner as an equipotential (isothermal) sphere. Thus, the need for dark matter or modified gravity theory is
mitigated, at least in part.
原版制作(carleton毕业证书)卡尔顿大学毕业证硕士文凭原版一模一样

原版纸张【微信:741003700 】【(carleton毕业证书)卡尔顿大学毕业证】【微信:741003700 】学位证,留信认证(真实可查,永久存档)offer、雅思、外壳等材料/诚信可靠,可直接看成品样本,帮您解决无法毕业带来的各种难题!外壳,原版制作,诚信可靠,可直接看成品样本。行业标杆!精益求精,诚心合作,真诚制作!多年品质 ,按需精细制作,24小时接单,全套进口原装设备。十五年致力于帮助留学生解决难题,包您满意。
本公司拥有海外各大学样板无数,能完美还原海外各大学 Bachelor Diploma degree, Master Degree Diploma
1:1完美还原海外各大学毕业材料上的工艺:水印,阴影底纹,钢印LOGO烫金烫银,LOGO烫金烫银复合重叠。文字图案浮雕、激光镭射、紫外荧光、温感、复印防伪等防伪工艺。材料咨询办理、认证咨询办理请加学历顾问Q/微741003700
留信网认证的作用:
1:该专业认证可证明留学生真实身份
2:同时对留学生所学专业登记给予评定
3:国家专业人才认证中心颁发入库证书
4:这个认证书并且可以归档倒地方
5:凡事获得留信网入网的信息将会逐步更新到个人身份内,将在公安局网内查询个人身份证信息后,同步读取人才网入库信息
6:个人职称评审加20分
7:个人信誉贷款加10分
8:在国家人才网主办的国家网络招聘大会中纳入资料,供国家高端企业选择人才
Compexometric titration/Chelatorphy titration/chelating titration

Classification
Metal ion ion indicators
Masking and demasking reagents
Estimation of Magnisium sulphate
Calcium gluconate
Complexometric Titration/ chelatometry titration/chelating titration, introduction, Types-
1.Direct Titration
2.Back Titration
3.Replacement Titration
4.Indirect Titration
Masking agent, Demasking agents
formation of complex
comparition between masking and demasking agents,
Indicators/Metal ion indicators/ Metallochromic indicators/pM indicators,
Visual Technique,PM indicators (metallochromic), Indicators of pH, Redox Indicators
Instrumental Techniques-Photometry
Potentiometry
Miscellaneous methods.
Complex titration with EDTA.
SAR of Medicinal Chemistry 1st by dk.pdf

In this presentation include the prototype drug SAR on thus or with their examples .
Syllabus of Second Year B. Pharmacy
2019 PATTERN.
Thornton ESPP slides UK WW Network 4_6_24.pdf

ESPP presentation to EU Waste Water Network, 4th June 2024 “EU policies driving nutrient removal and recycling
and the revised UWWTD (Urban Waste Water Treatment Directive)”
Sharlene Leurig - Enabling Onsite Water Use with Net Zero Water

Sharlene Leurig - Enabling Onsite Water Use with Net Zero WaterTexas Alliance of Groundwater Districts
Presented at June 6-7 Texas Alliance of Groundwater Districts Business MeetingESR spectroscopy in liquid food and beverages.pptx

With increasing population, people need to rely on packaged food stuffs. Packaging of food materials requires the preservation of food. There are various methods for the treatment of food to preserve them and irradiation treatment of food is one of them. It is the most common and the most harmless method for the food preservation as it does not alter the necessary micronutrients of food materials. Although irradiated food doesn’t cause any harm to the human health but still the quality assessment of food is required to provide consumers with necessary information about the food. ESR spectroscopy is the most sophisticated way to investigate the quality of the food and the free radicals induced during the processing of the food. ESR spin trapping technique is useful for the detection of highly unstable radicals in the food. The antioxidant capability of liquid food and beverages in mainly performed by spin trapping technique.
THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...

THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...Abdul Wali Khan University Mardan,kP,Pakistan
hematic appreciation test is a psychological assessment tool used to measure an individual's appreciation and understanding of specific themes or topics. This test helps to evaluate an individual's ability to connect different ideas and concepts within a given theme, as well as their overall comprehension and interpretation skills. The results of the test can provide valuable insights into an individual's cognitive abilities, creativity, and critical thinking skillsAuthoring a personal GPT for your research and practice: How we created the Q...

Thematic analysis in qualitative research is a time-consuming and systematic task, typically done using teams. Team members must ground their activities on common understandings of the major concepts underlying the thematic analysis, and define criteria for its development. However, conceptual misunderstandings, equivocations, and lack of adherence to criteria are challenges to the quality and speed of this process. Given the distributed and uncertain nature of this process, we wondered if the tasks in thematic analysis could be supported by readily available artificial intelligence chatbots. Our early efforts point to potential benefits: not just saving time in the coding process but better adherence to criteria and grounding, by increasing triangulation between humans and artificial intelligence. This tutorial will provide a description and demonstration of the process we followed, as two academic researchers, to develop a custom ChatGPT to assist with qualitative coding in the thematic data analysis process of immersive learning accounts in a survey of the academic literature: QUAL-E Immersive Learning Thematic Analysis Helper. In the hands-on time, participants will try out QUAL-E and develop their ideas for their own qualitative coding ChatGPT. Participants that have the paid ChatGPT Plus subscription can create a draft of their assistants. The organizers will provide course materials and slide deck that participants will be able to utilize to continue development of their custom GPT. The paid subscription to ChatGPT Plus is not required to participate in this workshop, just for trying out personal GPTs during it.
Shallowest Oil Discovery of Turkiye.pptx

The Petroleum System of the Çukurova Field - the Shallowest Oil Discovery of Türkiye, Adana
Travis Hills' Endeavors in Minnesota: Fostering Environmental and Economic Pr...

Travis Hills of Minnesota developed a method to convert waste into high-value dry fertilizer, significantly enriching soil quality. By providing farmers with a valuable resource derived from waste, Travis Hills helps enhance farm profitability while promoting environmental stewardship. Travis Hills' sustainable practices lead to cost savings and increased revenue for farmers by improving resource efficiency and reducing waste.
Describing and Interpreting an Immersive Learning Case with the Immersion Cub...

Current descriptions of immersive learning cases are often difficult or impossible to compare. This is due to a myriad of different options on what details to include, which aspects are relevant, and on the descriptive approaches employed. Also, these aspects often combine very specific details with more general guidelines or indicate intents and rationales without clarifying their implementation. In this paper we provide a method to describe immersive learning cases that is structured to enable comparisons, yet flexible enough to allow researchers and practitioners to decide which aspects to include. This method leverages a taxonomy that classifies educational aspects at three levels (uses, practices, and strategies) and then utilizes two frameworks, the Immersive Learning Brain and the Immersion Cube, to enable a structured description and interpretation of immersive learning cases. The method is then demonstrated on a published immersive learning case on training for wind turbine maintenance using virtual reality. Applying the method results in a structured artifact, the Immersive Learning Case Sheet, that tags the case with its proximal uses, practices, and strategies, and refines the free text case description to ensure that matching details are included. This contribution is thus a case description method in support of future comparative research of immersive learning cases. We then discuss how the resulting description and interpretation can be leveraged to change immersion learning cases, by enriching them (considering low-effort changes or additions) or innovating (exploring more challenging avenues of transformation). The method holds significant promise to support better-grounded research in immersive learning.
waterlessdyeingtechnolgyusing carbon dioxide chemicalspdf

The technology uses reclaimed CO₂ as the dyeing medium in a closed loop process. When pressurized, CO₂ becomes supercritical (SC-CO₂). In this state CO₂ has a very high solvent power, allowing the dye to dissolve easily.
Recently uploaded (20)
aziz sancar nobel prize winner: from mardin to nobel

aziz sancar nobel prize winner: from mardin to nobel
Deep Software Variability and Frictionless Reproducibility

Deep Software Variability and Frictionless Reproducibility
The use of Nauplii and metanauplii artemia in aquaculture (brine shrimp).pptx

The use of Nauplii and metanauplii artemia in aquaculture (brine shrimp).pptx
The binding of cosmological structures by massless topological defects

The binding of cosmological structures by massless topological defects
mô tả các thí nghiệm về đánh giá tác động dòng khí hóa sau đốt

mô tả các thí nghiệm về đánh giá tác động dòng khí hóa sau đốt
Compexometric titration/Chelatorphy titration/chelating titration

Compexometric titration/Chelatorphy titration/chelating titration
Basics of crystallography, crystal systems, classes and different forms

Basics of crystallography, crystal systems, classes and different forms
Sharlene Leurig - Enabling Onsite Water Use with Net Zero Water

Sharlene Leurig - Enabling Onsite Water Use with Net Zero Water
ESR spectroscopy in liquid food and beverages.pptx

ESR spectroscopy in liquid food and beverages.pptx
THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...

THEMATIC APPERCEPTION TEST(TAT) cognitive abilities, creativity, and critic...
Authoring a personal GPT for your research and practice: How we created the Q...

Authoring a personal GPT for your research and practice: How we created the Q...
Travis Hills' Endeavors in Minnesota: Fostering Environmental and Economic Pr...

Travis Hills' Endeavors in Minnesota: Fostering Environmental and Economic Pr...
Describing and Interpreting an Immersive Learning Case with the Immersion Cub...

Describing and Interpreting an Immersive Learning Case with the Immersion Cub...
waterlessdyeingtechnolgyusing carbon dioxide chemicalspdf

waterlessdyeingtechnolgyusing carbon dioxide chemicalspdf
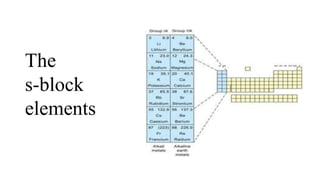
s block elements Part5.pptx
- 2. General characteristics of the compounds of the alkali metals All the common compounds of the alkali metals are generally ionic in nature. 1. Oxides and Hydroxides • On combustion in excess of air, lithium forms mainly the oxide, Li2O , sodium forms the peroxide, Na2O2 (and some superoxide NaO2) whilst potassium, rubidium and caesium form the superoxides, MO2. Under appropriate conditions pure compounds M2O, M2O2 and MO2 may be prepared. • The increasing stability of the peroxide or superoxide, as the size of the metal ion increases, is due to the stabilisation of large anions by larger cations through lattice energy effects.
- 3. • The oxides and the peroxides -- colourless (pure), • the superoxides -- yellow or orange in colour And paramagnetic • Sodium peroxide is widely used as an oxidising agent in inorganic chemistry. • The hydroxides -- all white crystalline solids. • The alkali metal hydroxides are the strongest of all bases and dissolve freely in water with evolution of much heat on account of intense hydration. • These oxides are easily hydrolysed by water to form the hydroxides according to the following reactions :
- 4. 2.Halides • The alkali metal halides, MX, (X=F,Cl,Br,I) are all high melting, colourless crystalline solids. • They can be prepared by the reaction of the appropriate oxide, hydroxide or carbonate with aqueous hydrohalic acid (HX). • All of these halides have high negative enthalpies of formation • For a given metal ∆f H-always becomes less negative from fluoride to iodide. • The melting and boiling points always follow the trend: fluoride > chloride > bromide > iodide. • All these halides are soluble in water. The low solubility of LiF in water is due to its high hydration enthalpy whereas the low solubility of CsI is due to smaller hydration enthalpy of its two ions.
- 5. 3.Oxo-acids are those in which the acidic proton is on a hydroxyl group with an oxo group attached to the same atom e.g., carbonic acid, H2CO3 ; sulphuric acid, H2SO4. The alkali metals form salts with all the oxo-acids. They are generally soluble in water and thermally stable.
- 6. • Their carbonates (M2CO3) and in most cases the hydrogencarbonates (MHCO3) also are highly stable to heat. • As the electropositive character increases down the group, the stability of the carbonates and hydorgencarbonates increases. • Lithium carbonate is not so stable to heat; lithium being very small in size polarises a large CO3 2– ion leading to the formation of more stable Li2O and CO2. Its hydrogencarbonate does not exist as a solid
- 7. SOME IMPORTANT COMPOUNDS OF SODIUM Sodium Carbonate (Washing Soda), Na2CO3·10H2O • Sodium carbonate is generally prepared by Solvay Process. • In this process, as sodium hydrogencarbonate has low solubility it gets precipitated in the reaction of sodium chloride with ammonium hydrogencarbonate. • The latter is prepared by passing CO2 to a concentrated solution of sodium chloride saturated with ammonia, where ammonium carbonate followed by ammonium hydrogencarbonate are formed. The equations for the complete process may be written as :
- 8. Sodium hydrogencarbonate crystal separates. These are heated to give sodium carbonate In this process NH3 is recovered when the solution containing NH4Cl is treated with Ca(OH)2. Calcium chloride is obtained as a by-product. Solvay process cannot be used to manufacture potassium carbonate because, potassium hydrogencarbonate is too soluble to be precipitated.
- 9. Properties : • Sodium carbonate is a white crystalline solid which exists as a decahydrate, Na2CO3·10H2O. • This is also called washing soda. • It is readily soluble in water. • On heating, the decahydrate loses its water of crystallisation to form monohydrate. Above 373K, the monohydrate becomes completely anhydrous and changes to a white powder called soda ash. • Carbonate part of sodium carbonate gets hydrolysed by water to form an alkaline solution
- 10. Uses: (i) It is used in water softening, laundering and cleaning. (ii) It is used in the manufacture of glass, soap, borax and caustic soda. (iii) It is used in paper, paints and textile industries. (iv) It is an important laboratory reagent both in qualitative and quantitative analysis.