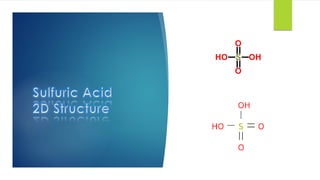
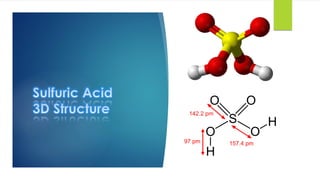
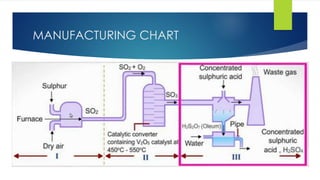
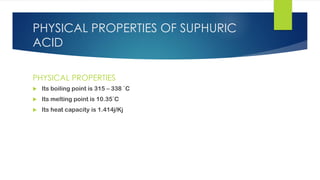
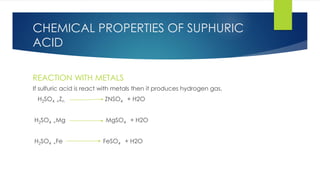
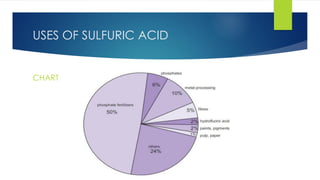
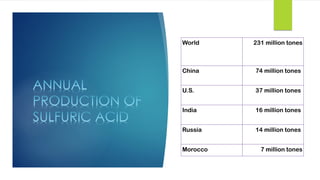
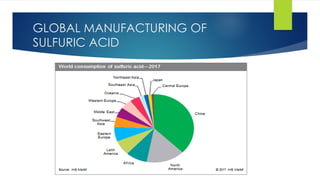
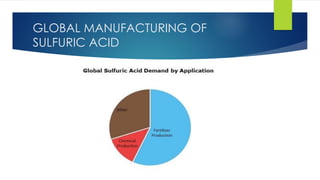
The document discusses sulfuric acid, including its chemical formula, properties, and manufacturing processes. It provides details on the contact process, which involves burning sulfur to produce SO2, purifying the SO2, oxidizing it to SO3, absorbing the SO3 in sulfuric acid to form oleum, and diluting the oleum. Sulfuric acid is a strong acid that is corrosive and widely used to make fertilizers and other chemicals. The largest global producers are China, the US, India, and Russia.