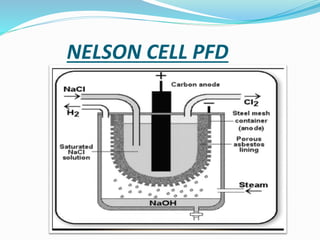
Sodium hydroxide was discovered in 1807 by Humphrey Day in England. It is a white solid compound consisting of sodium and hydroxide ions. It is produced industrially through electrolysis of brine using the Castner-Kellner or Nelson cell processes. Sodium hydroxide is very basic and has many industrial uses such as in soap production, rayon manufacturing, and petroleum products. It has important chemical properties like reacting with acids to form salts and water. Major sodium hydroxide producers in Pakistan include Sitara Chemicals, Tufail Chemicals, and ICI Pakistan.