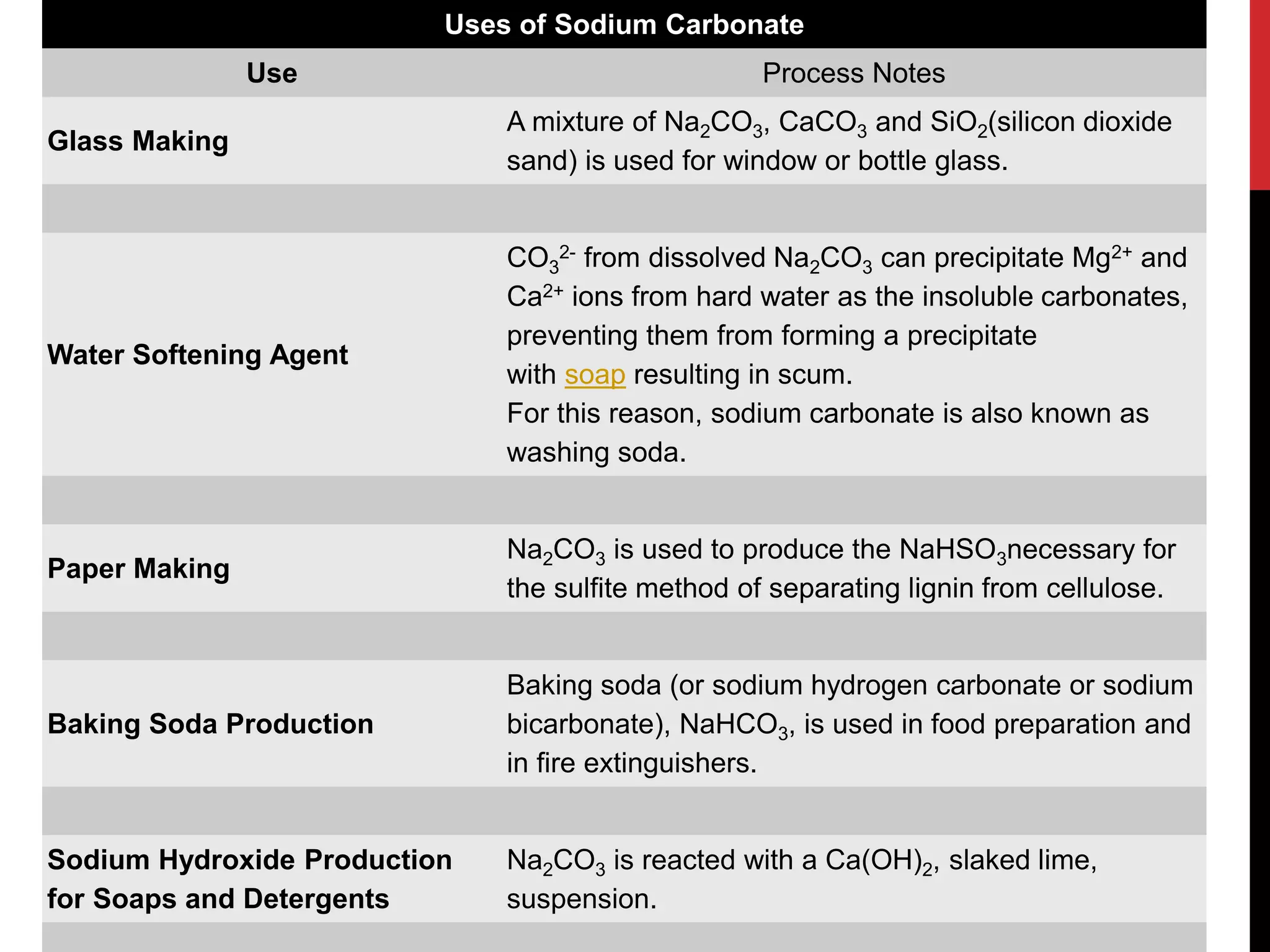
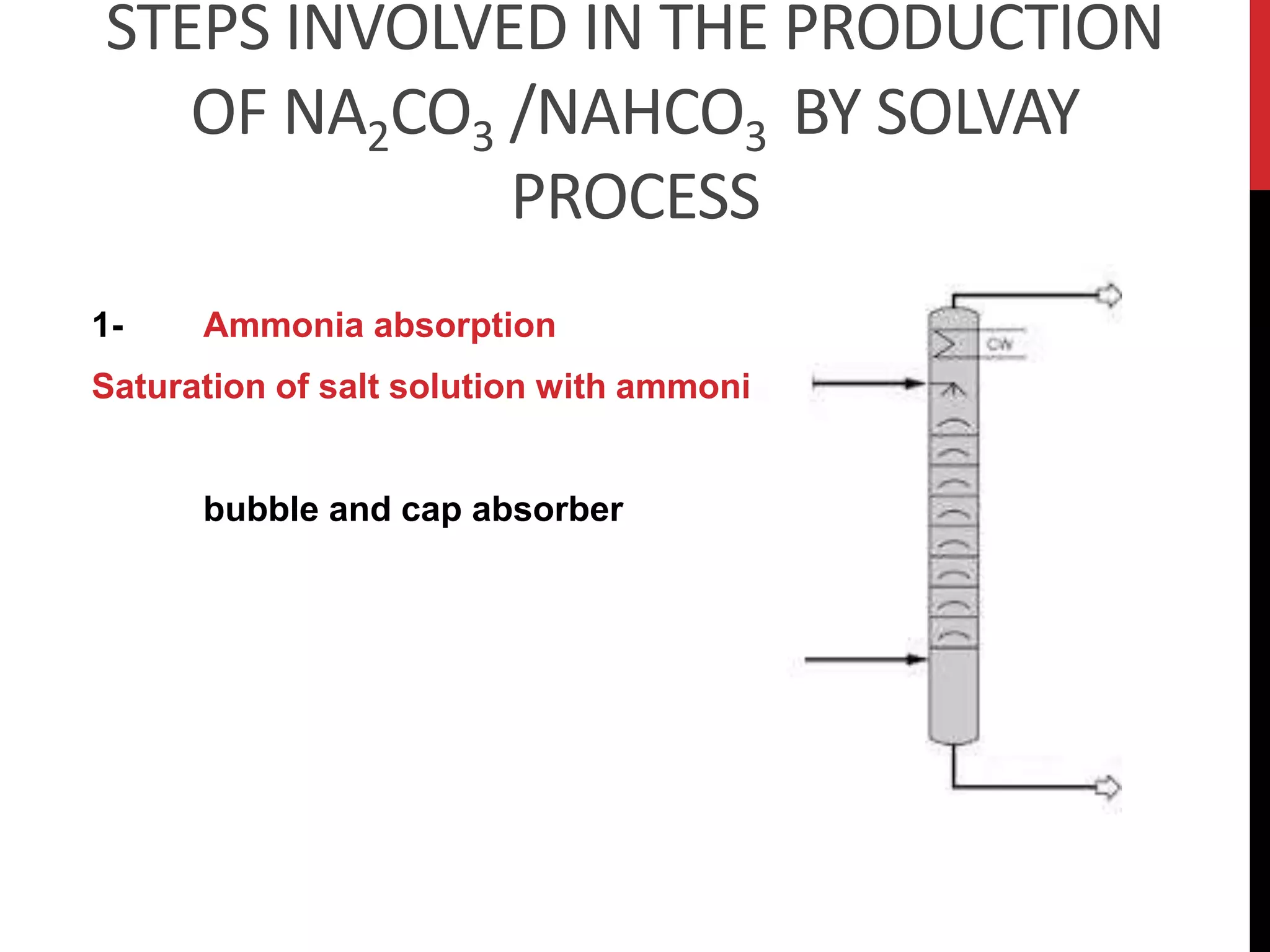
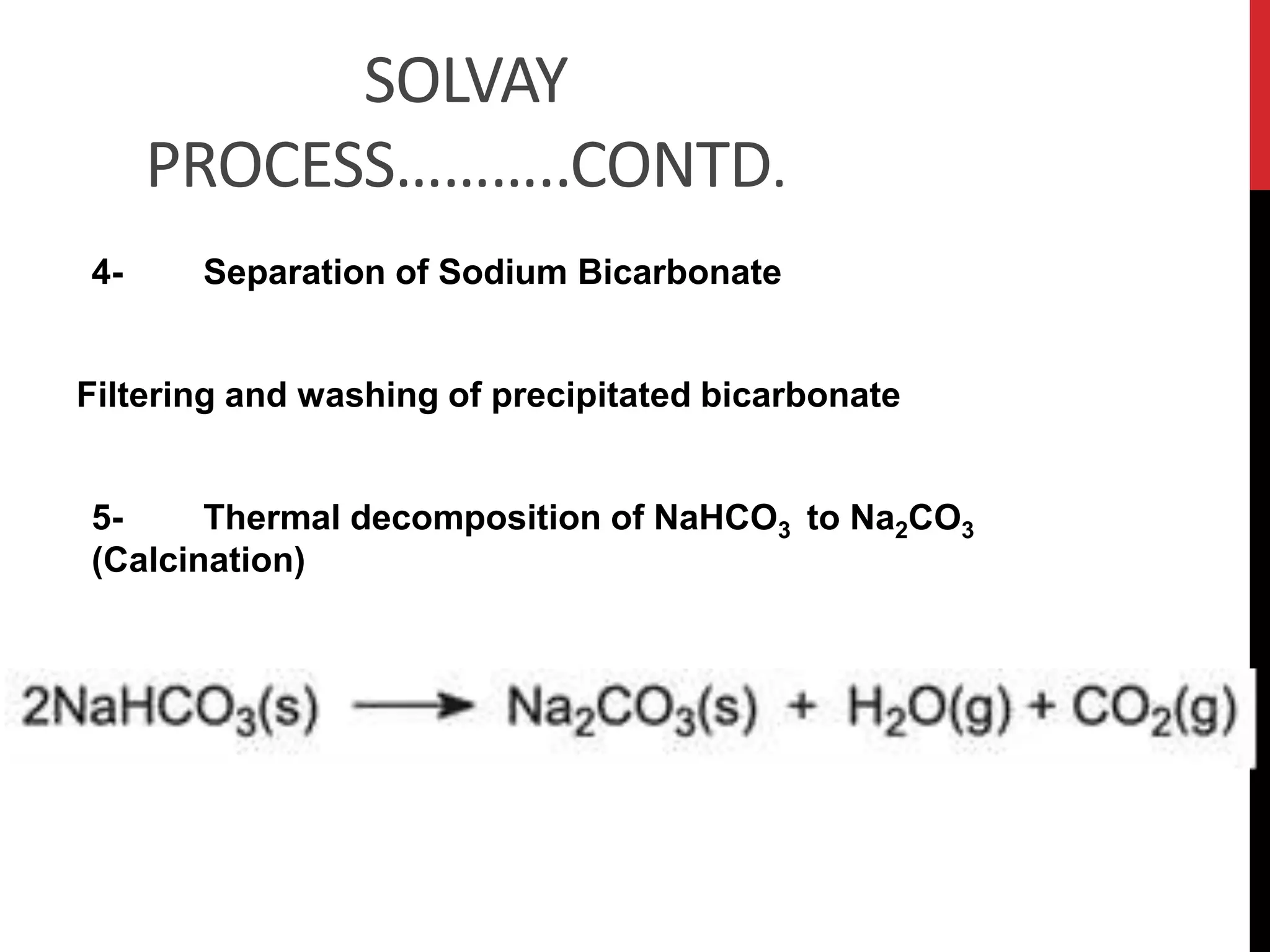
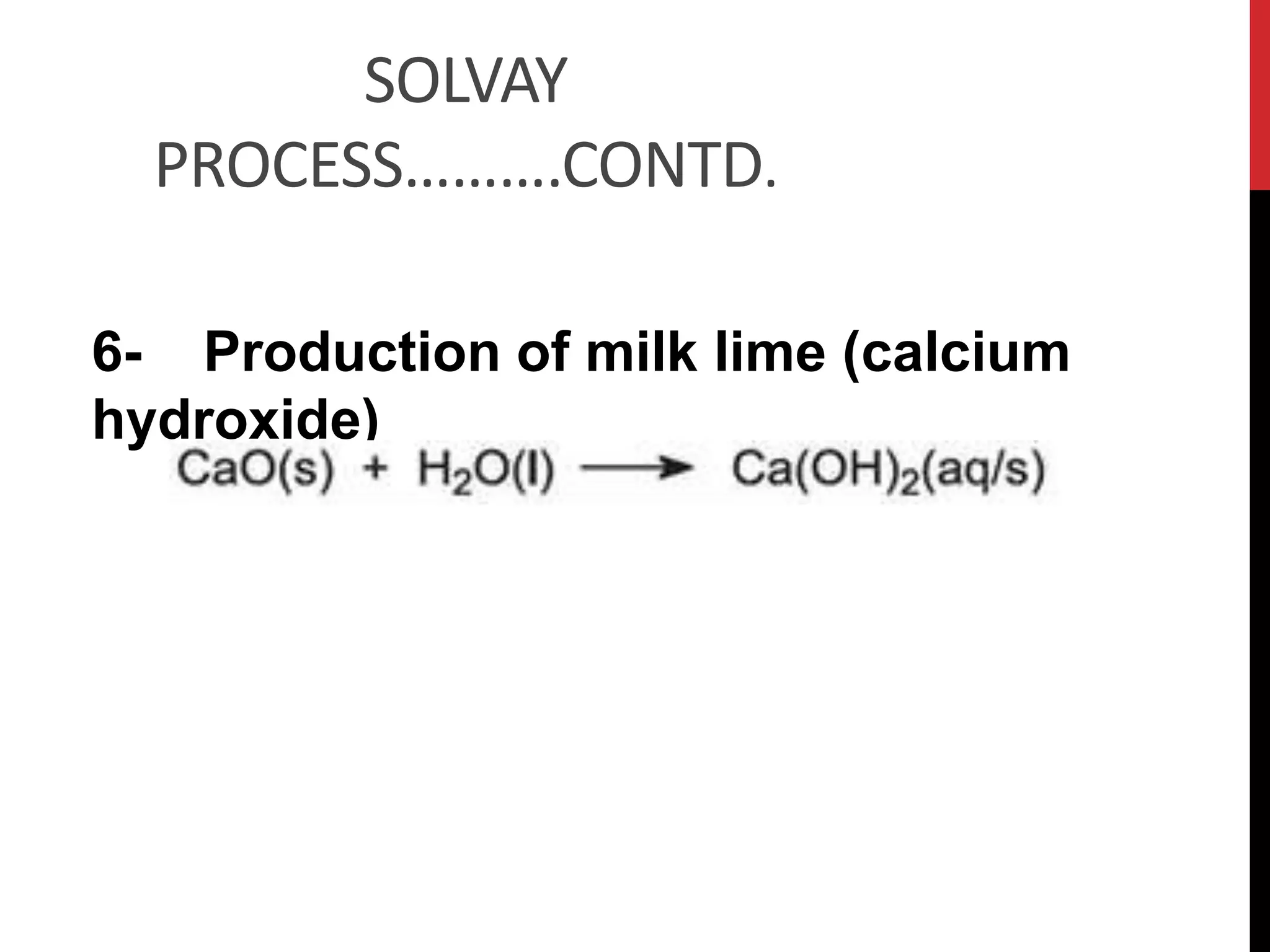
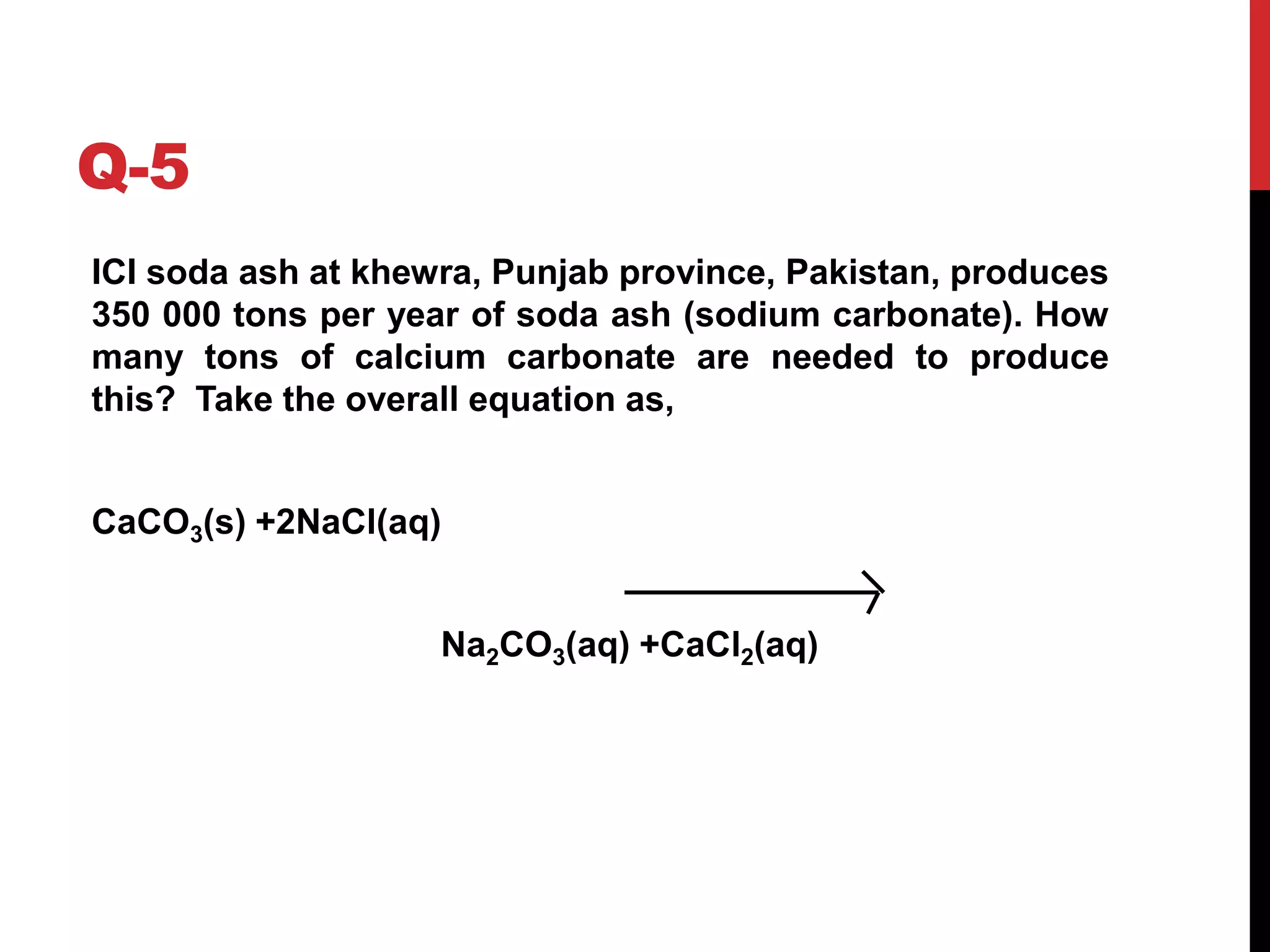
The document discusses the production of sodium carbonate and baking soda via the Solvay process. It begins with an overview of the uses of sodium carbonate and its raw materials. It then provides the overall reaction and a process flow diagram depicting the major steps. These steps include: 1) ammonia absorption into salt water, 2) production of carbon dioxide from limestone, 3) carbonation to form sodium bicarbonate, 4) thermal decomposition to produce sodium carbonate, 5) regeneration of ammonia. The document concludes with some questions about the process.