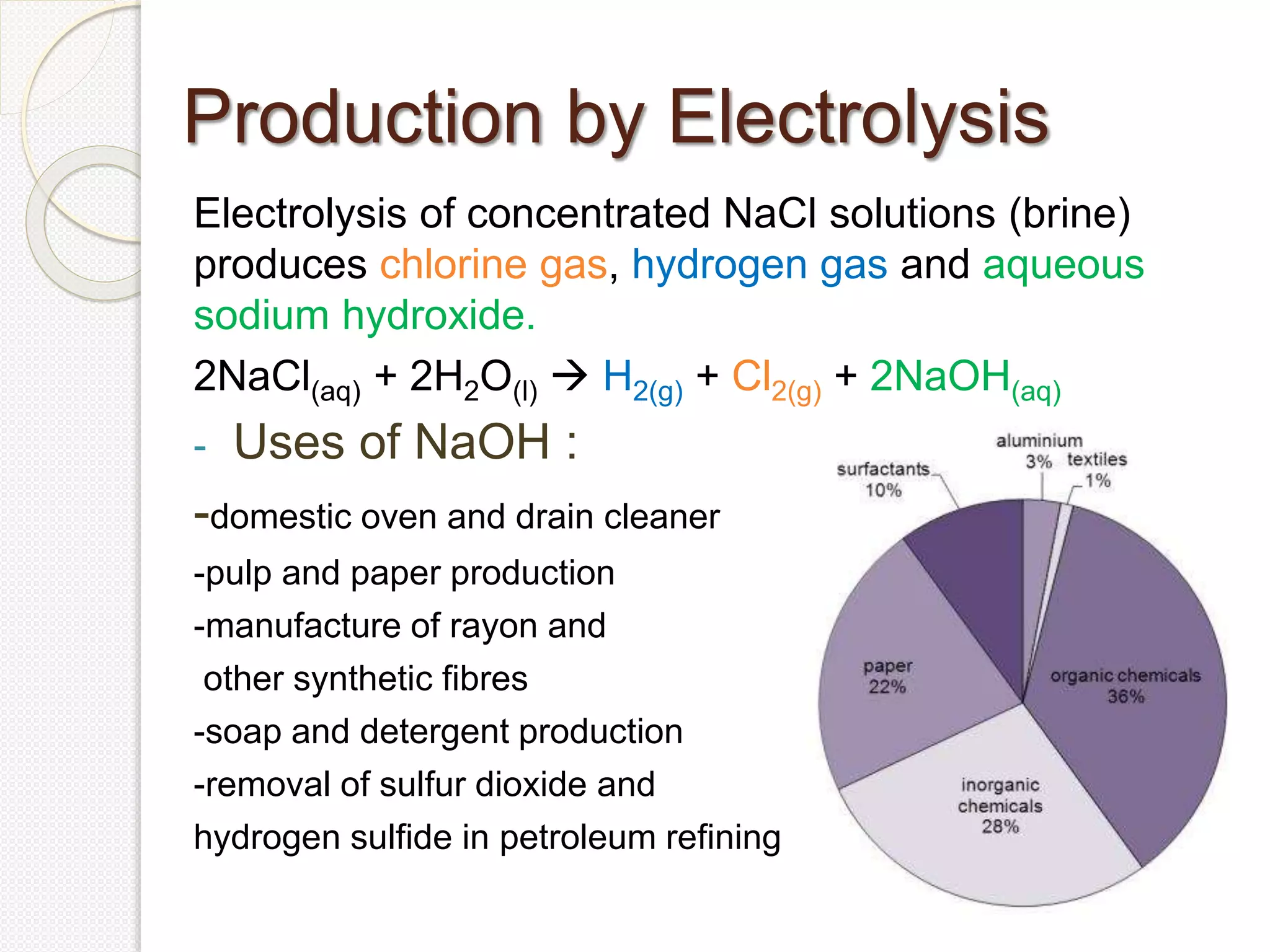
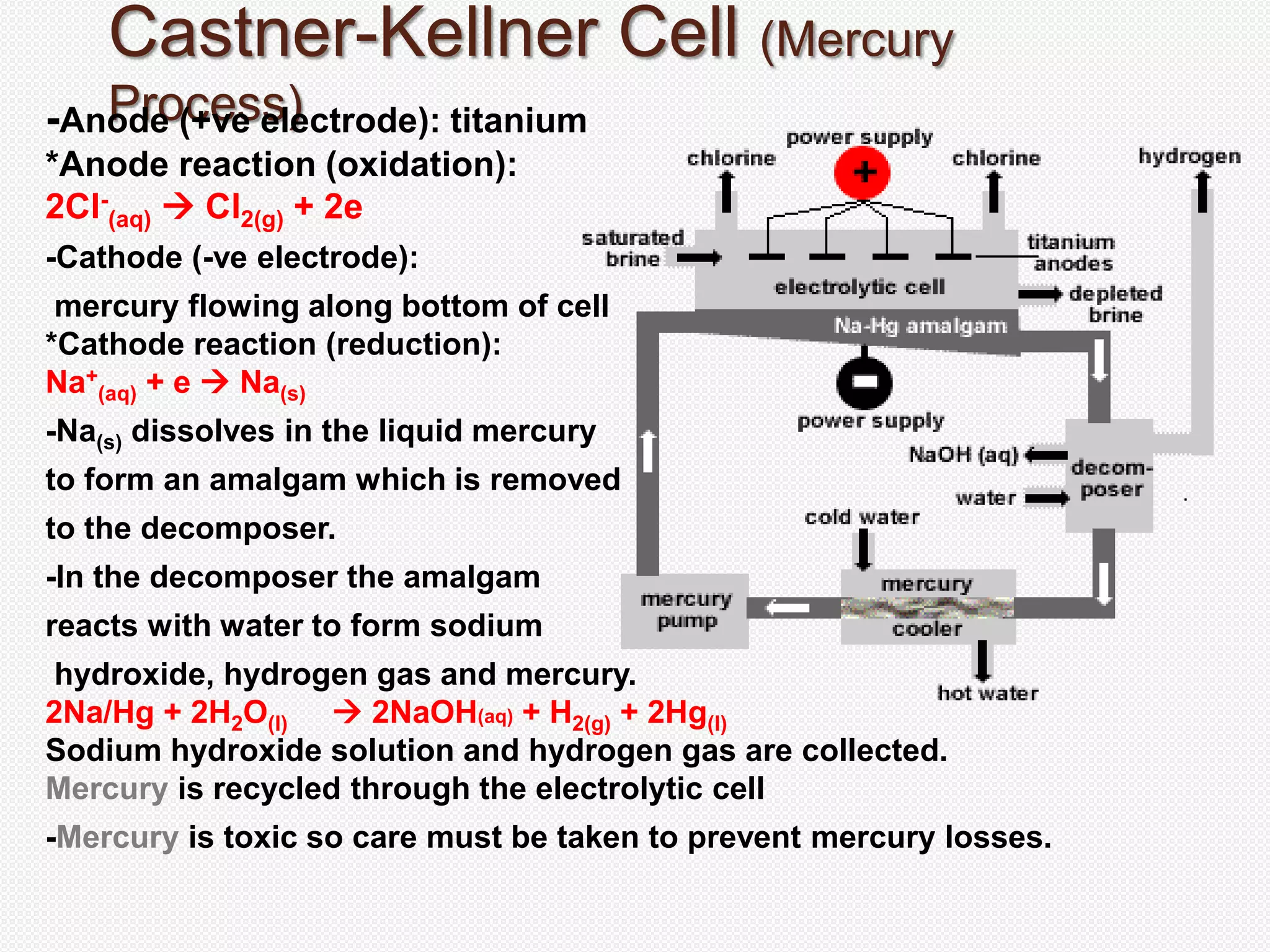
The document provides an overview of sodium hydroxide (NaOH), including its chemical properties, production methods via electrolysis of brine, and various applications such as household cleaners and industrial processes. It describes three electrolytic cells used for its production: the Castner-Kellner cell, Nelson diaphragm cell, and membrane cell, detailing their operations and advantages. Additionally, the document highlights the toxicity of chlorine gas and the environmental impact of certain production methods.