Embed presentation
Download to read offline





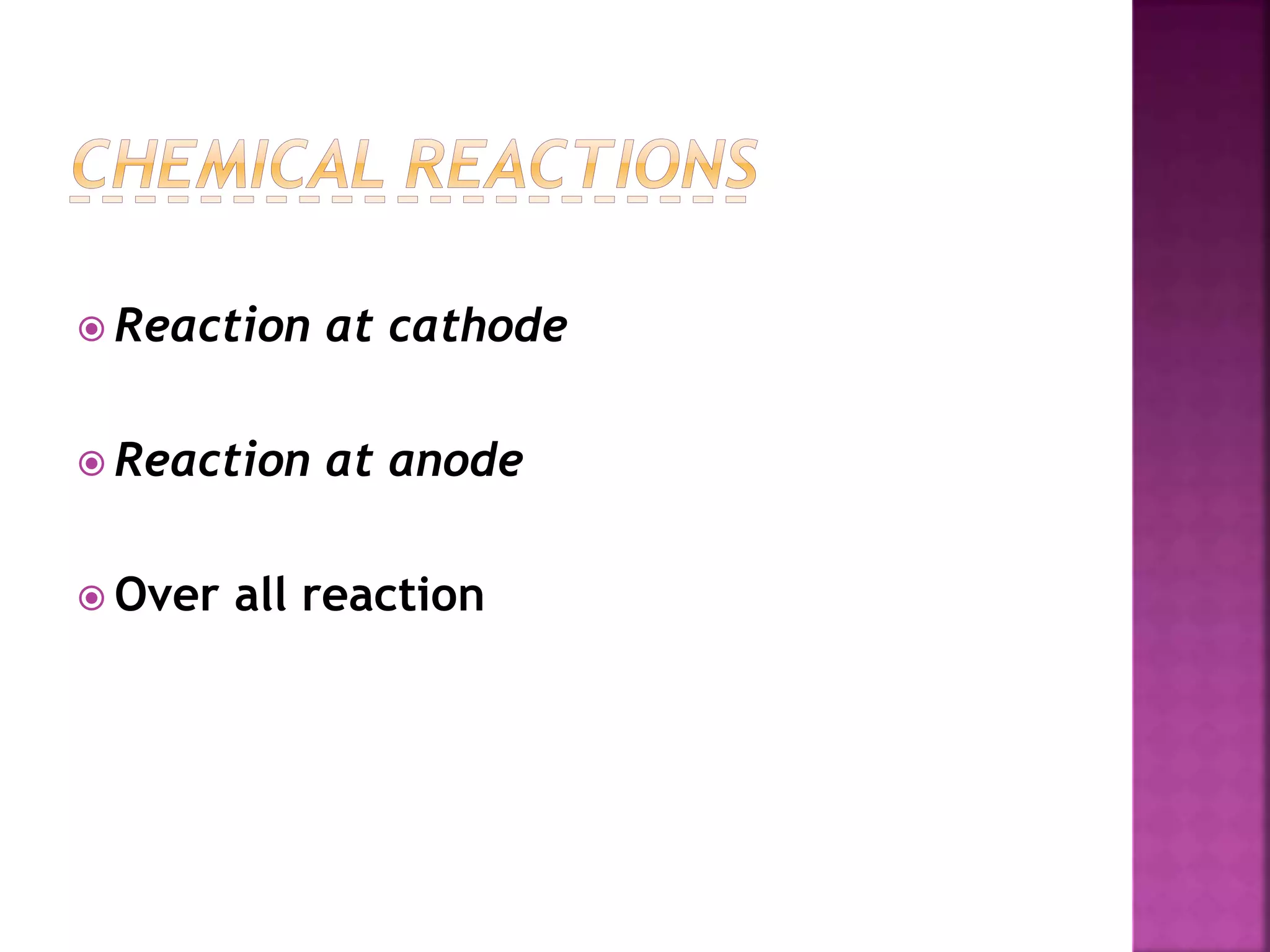
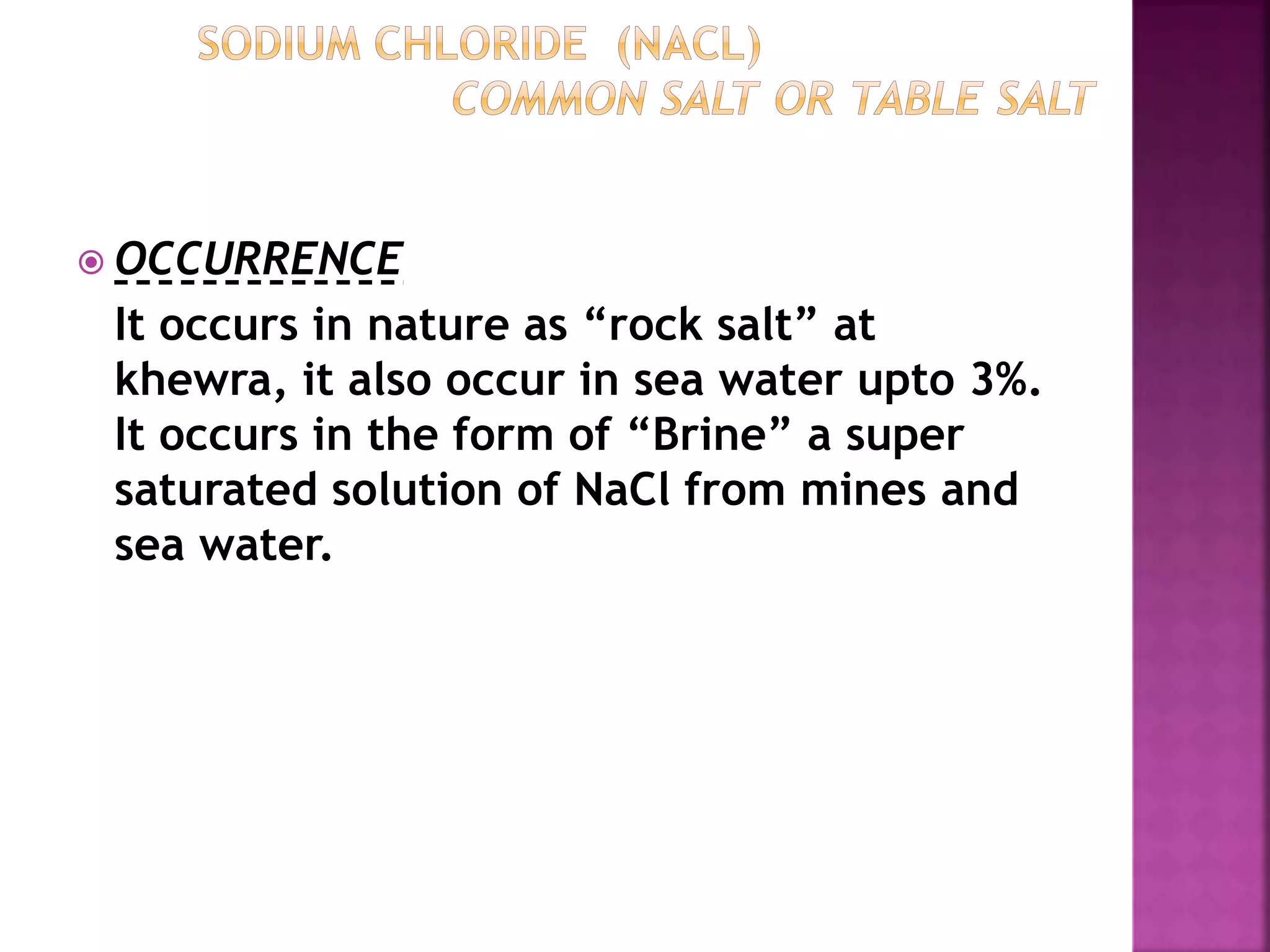







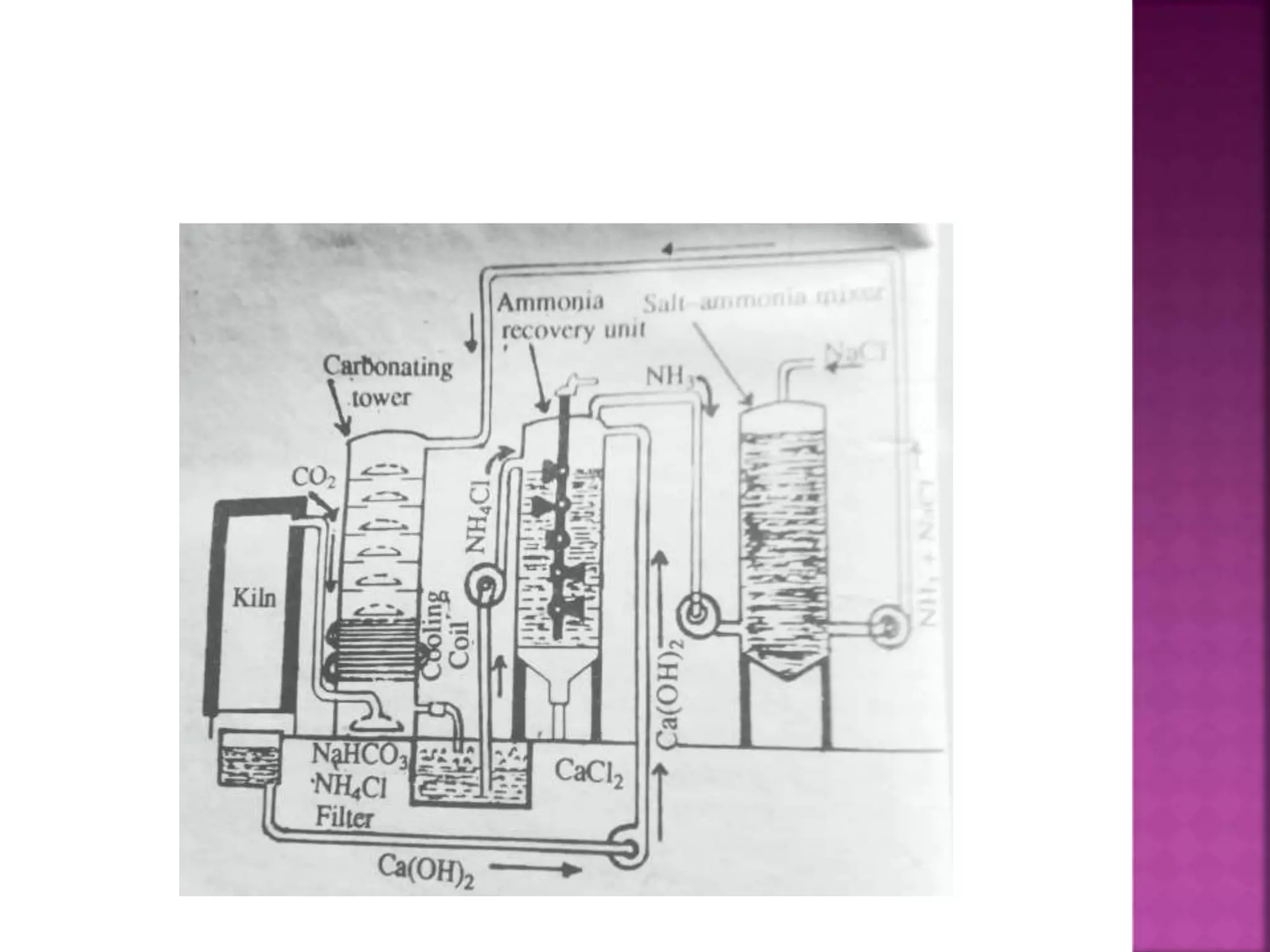
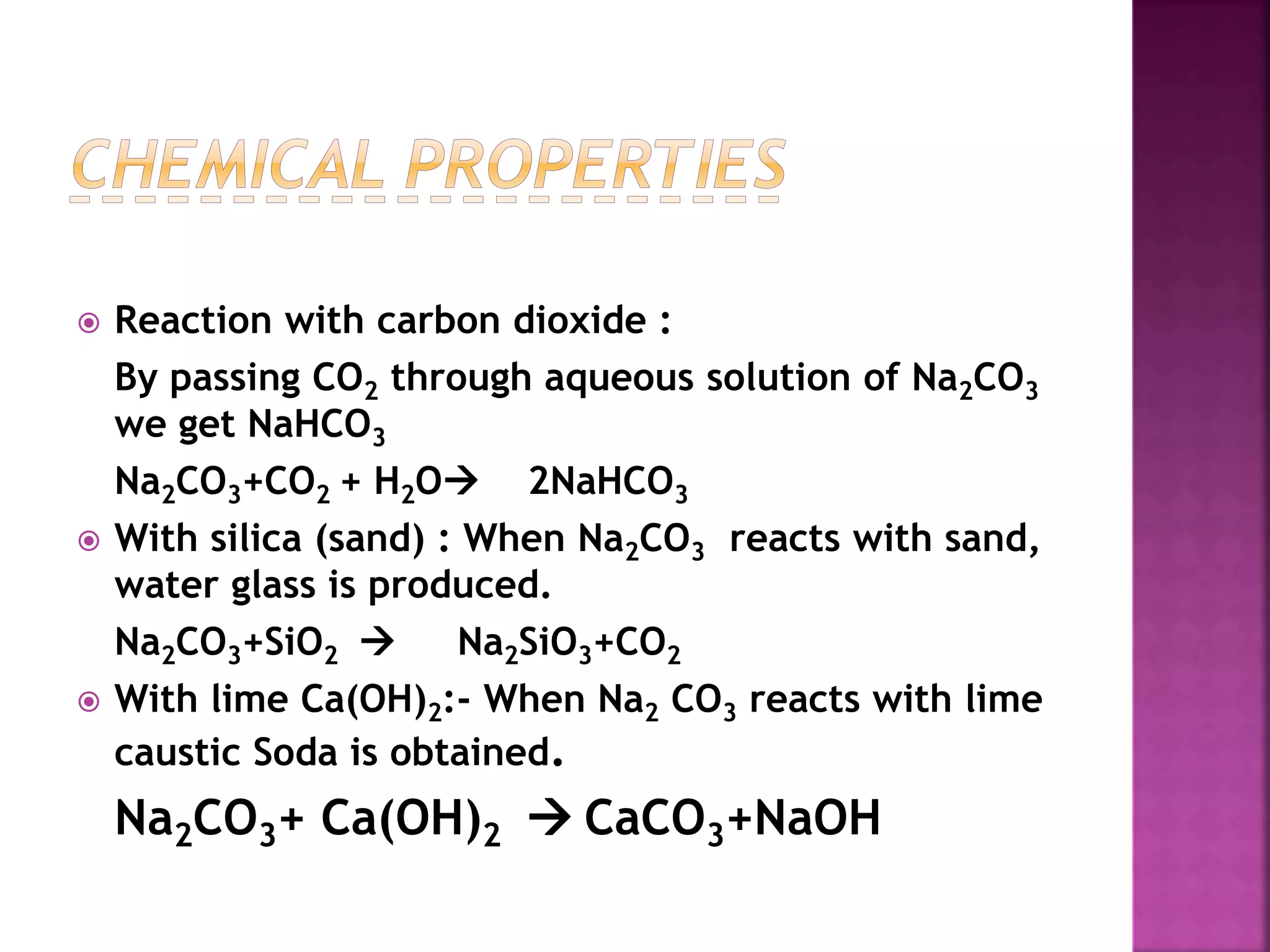

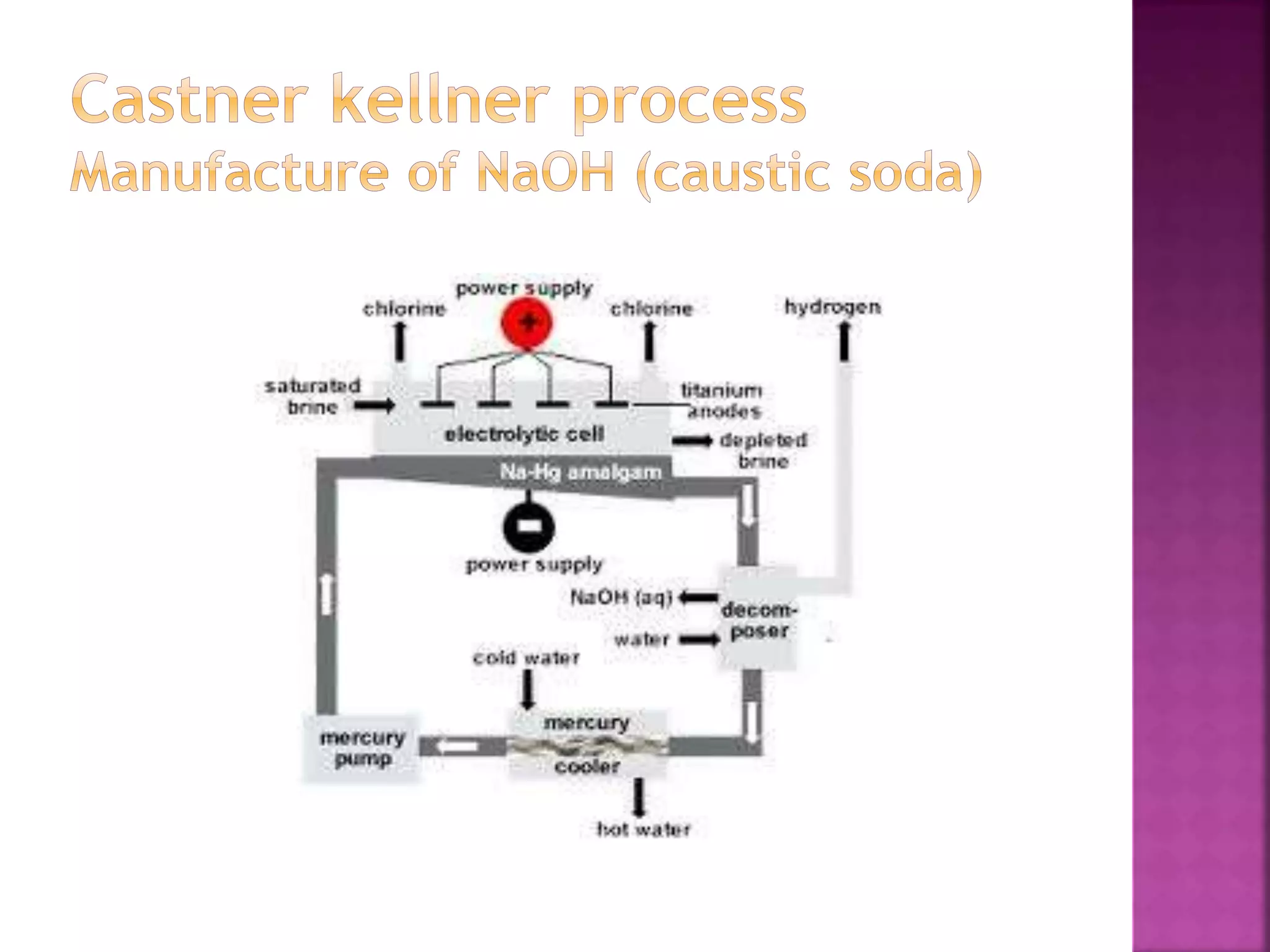
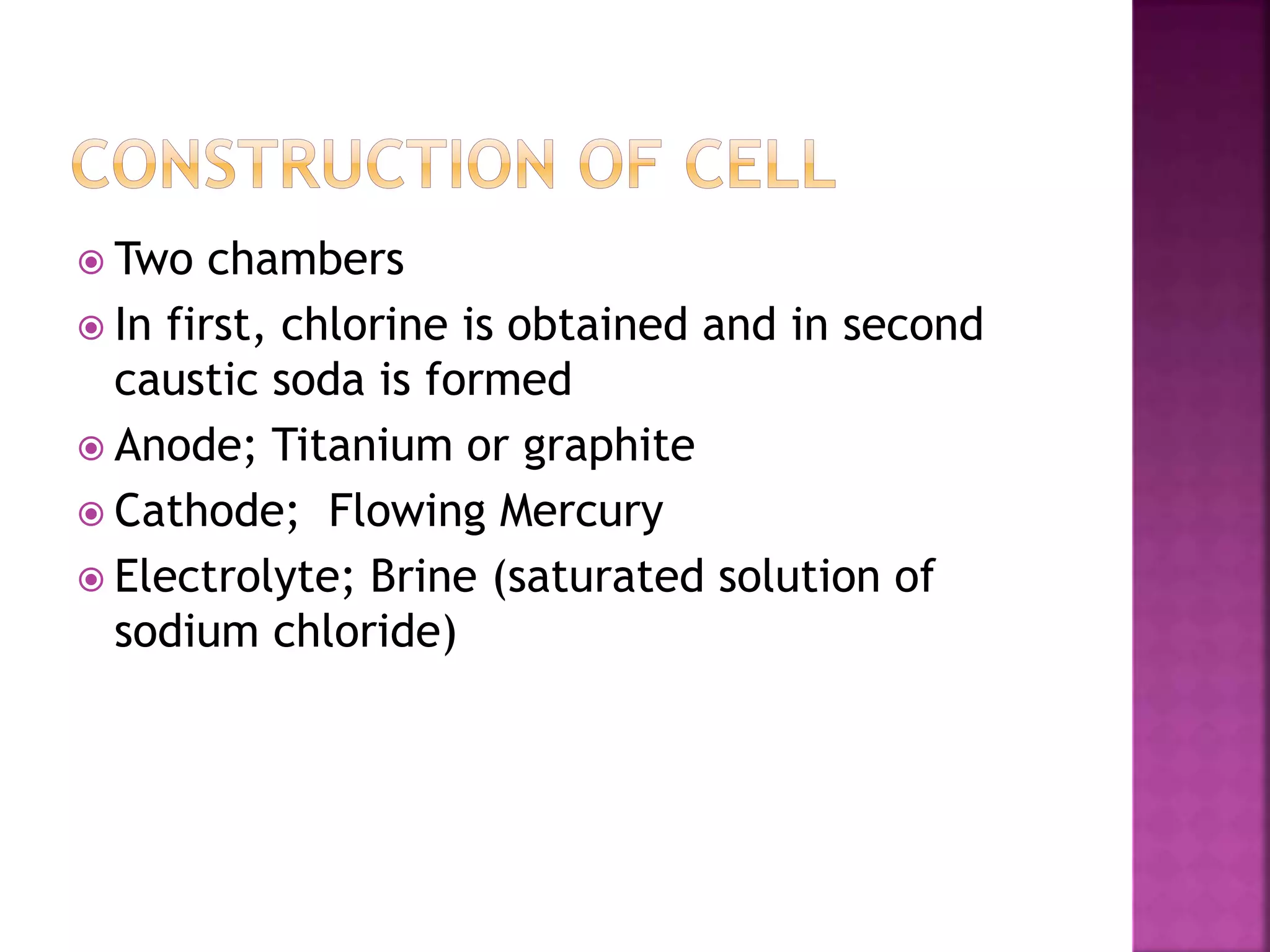
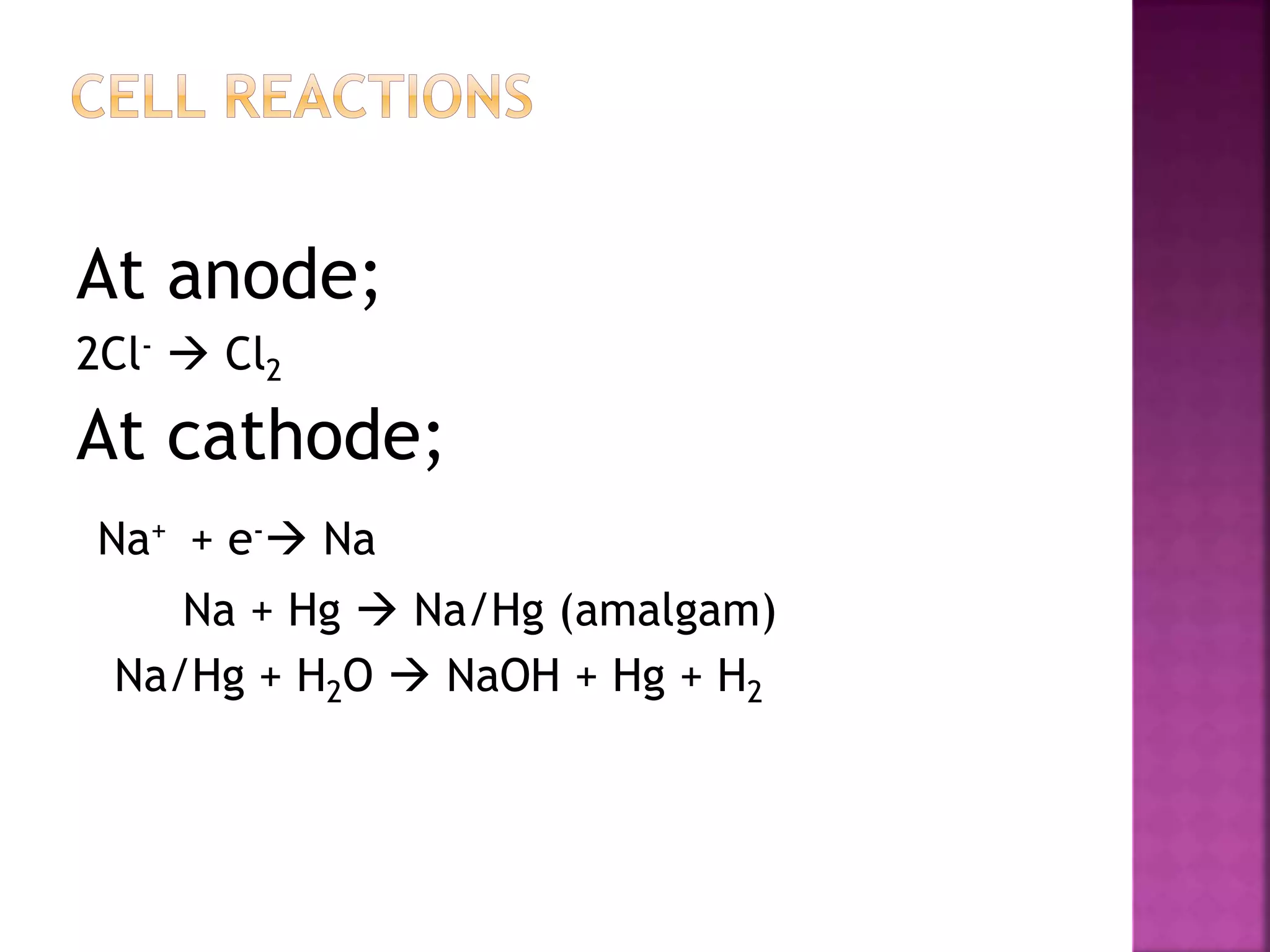






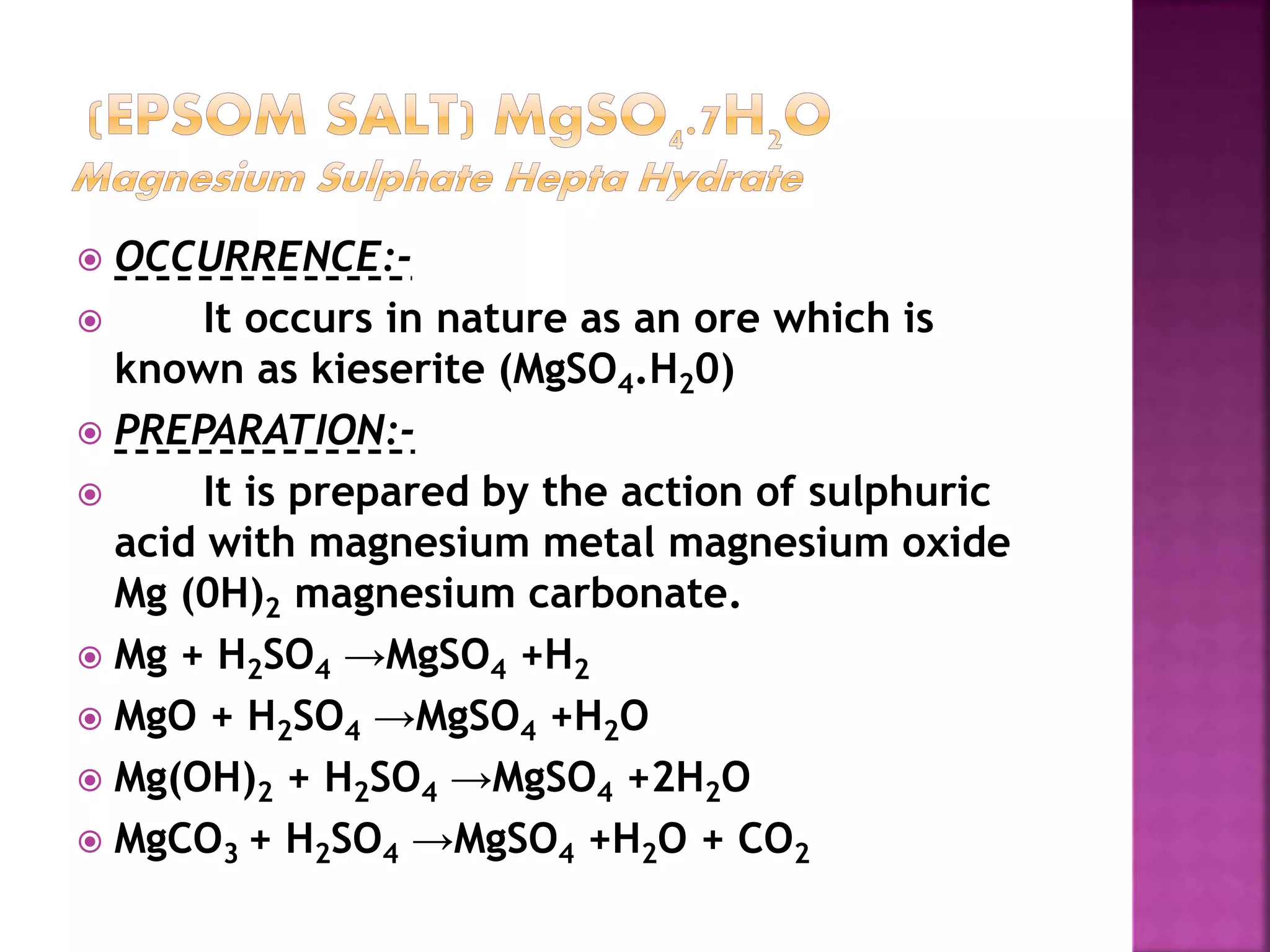







This document discusses the manufacture and properties of sodium and several other compounds. It describes the electrolysis process used to extract sodium from molten NaCl, lowering the melting point with CaCl2. Sodium is collected at the cathode and chlorine at the anode. The document also summarizes processes for manufacturing sodium carbonate, sodium hydroxide, magnesium sulfate, gypsum, plaster of paris, and bleaching powder. Their properties and uses are provided.
































