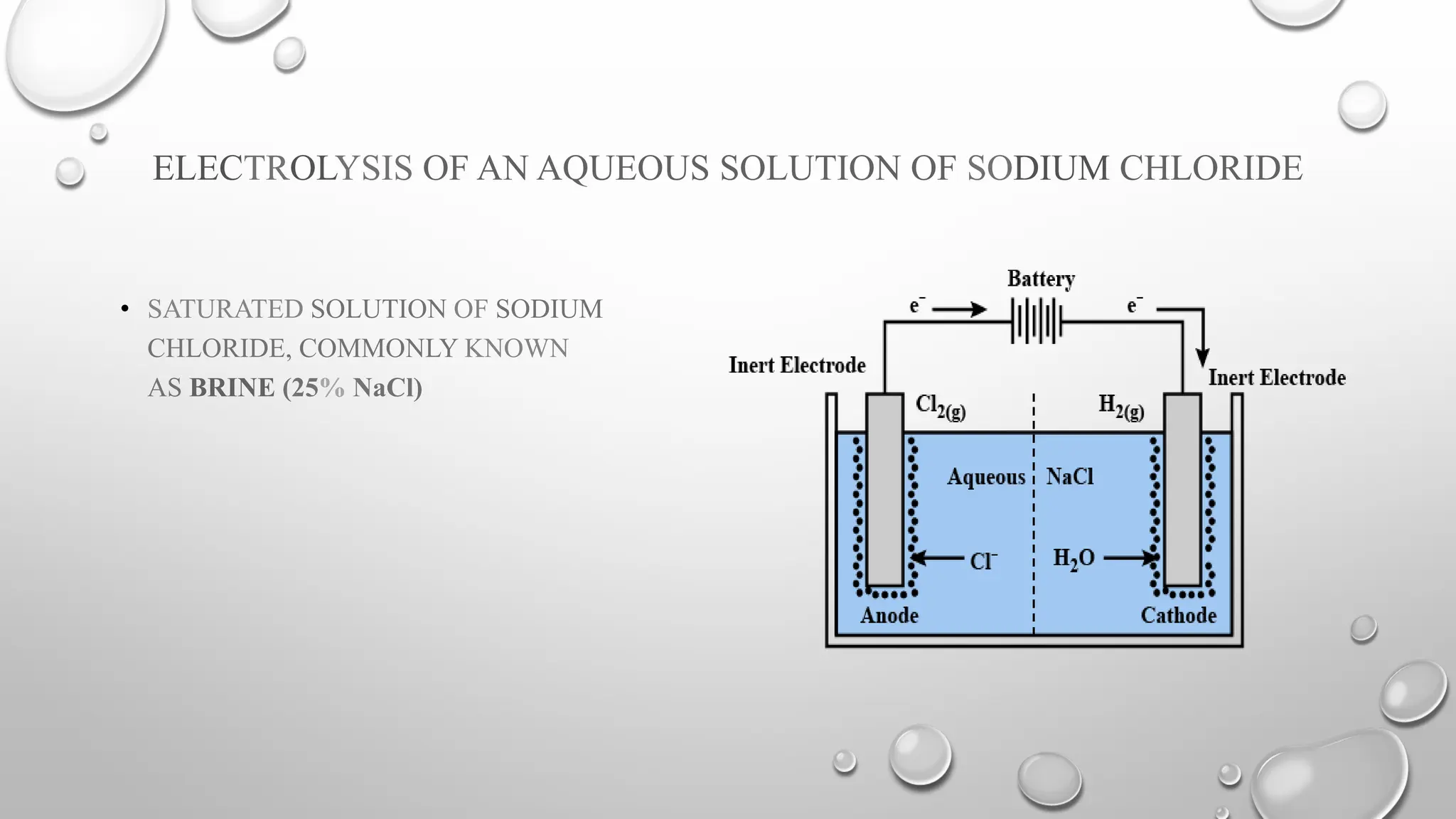
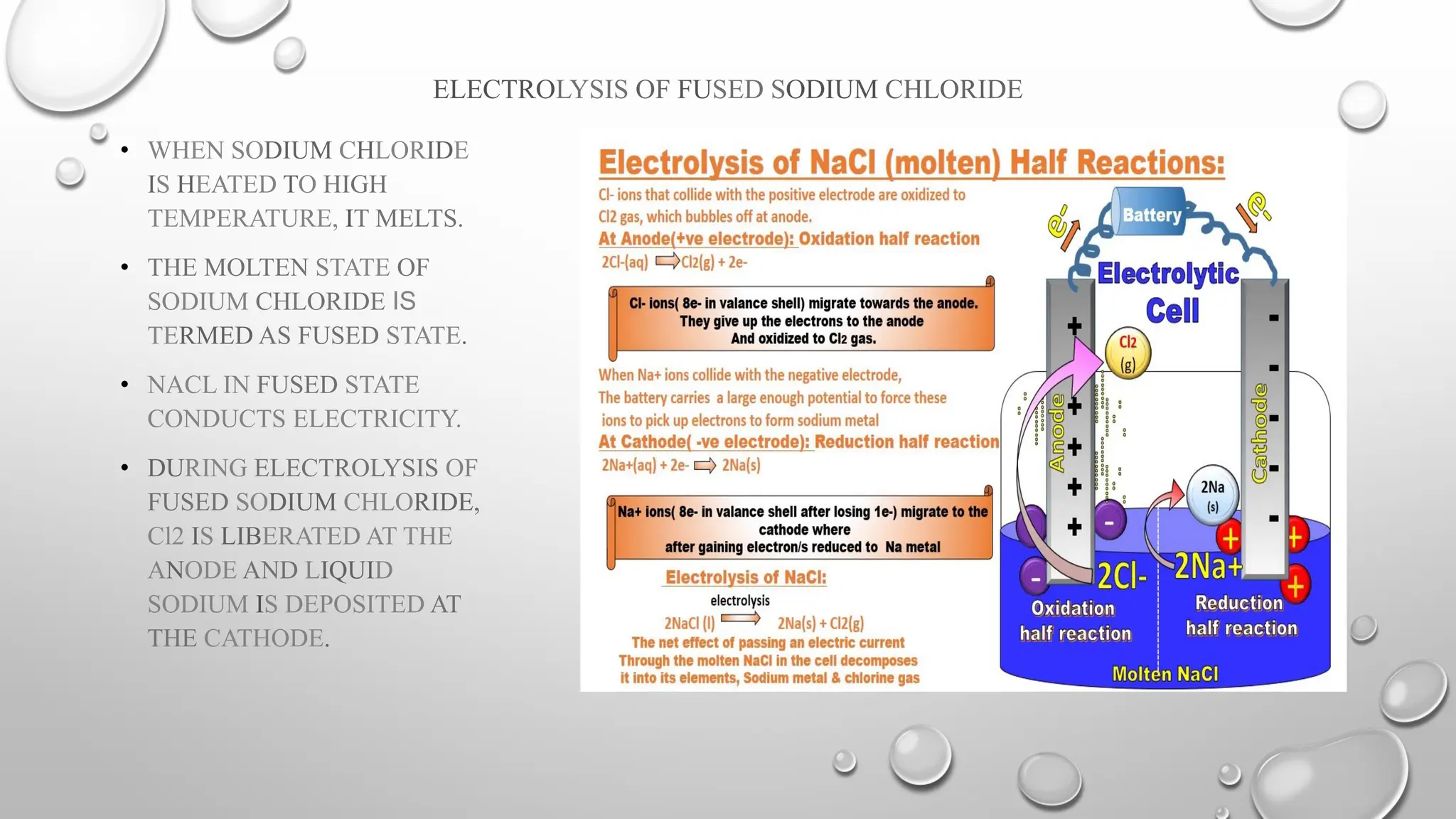
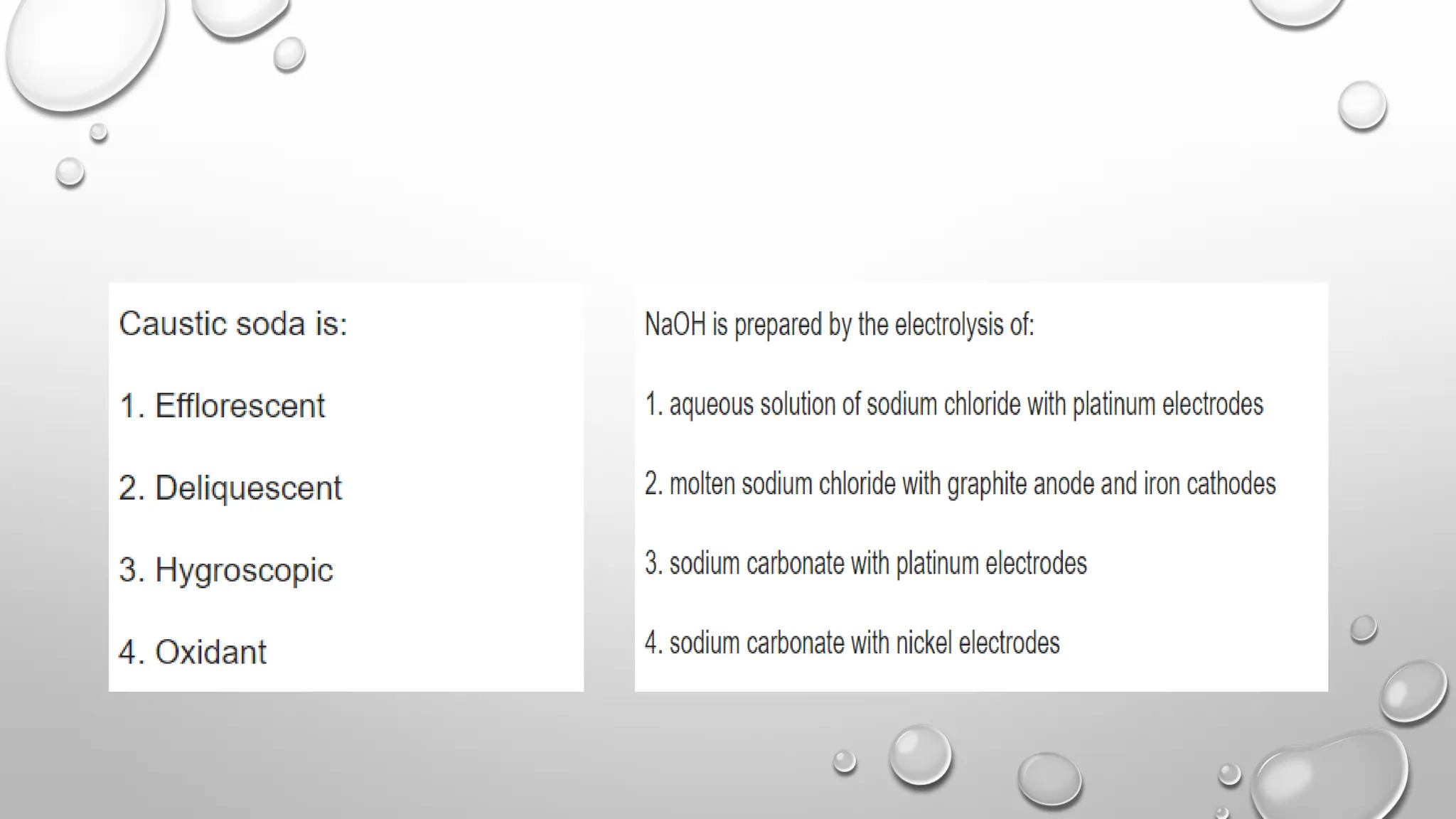
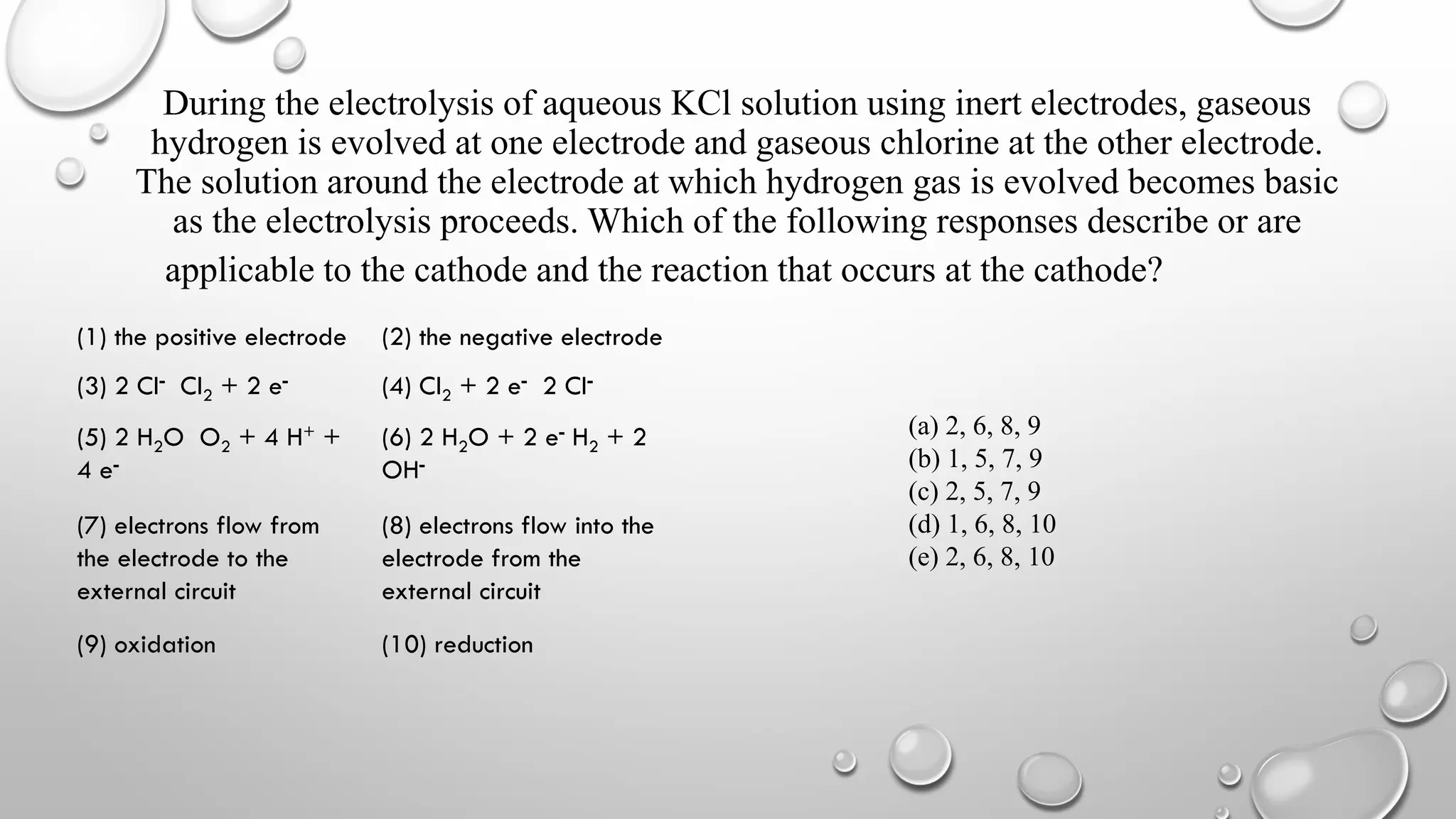
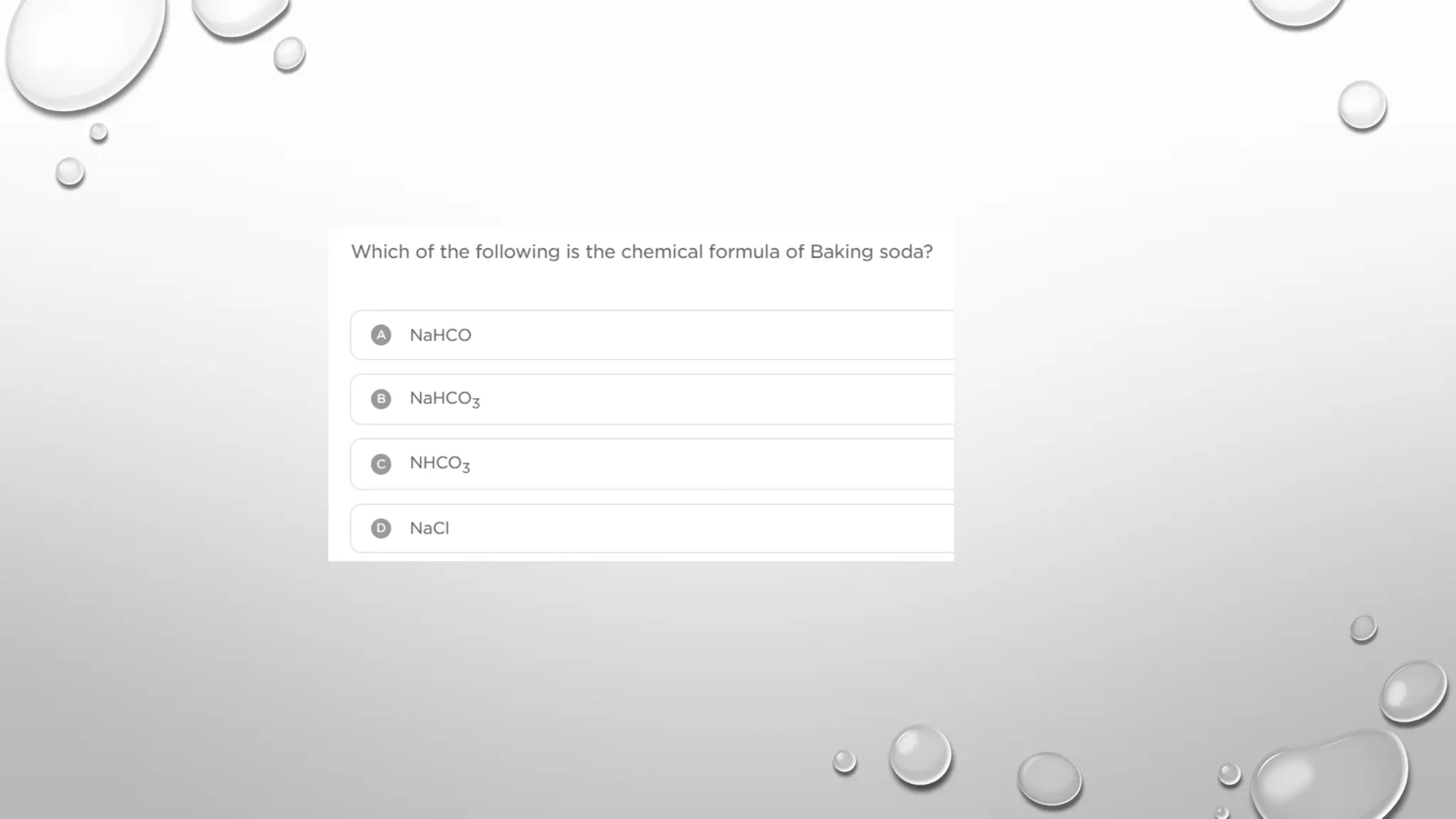
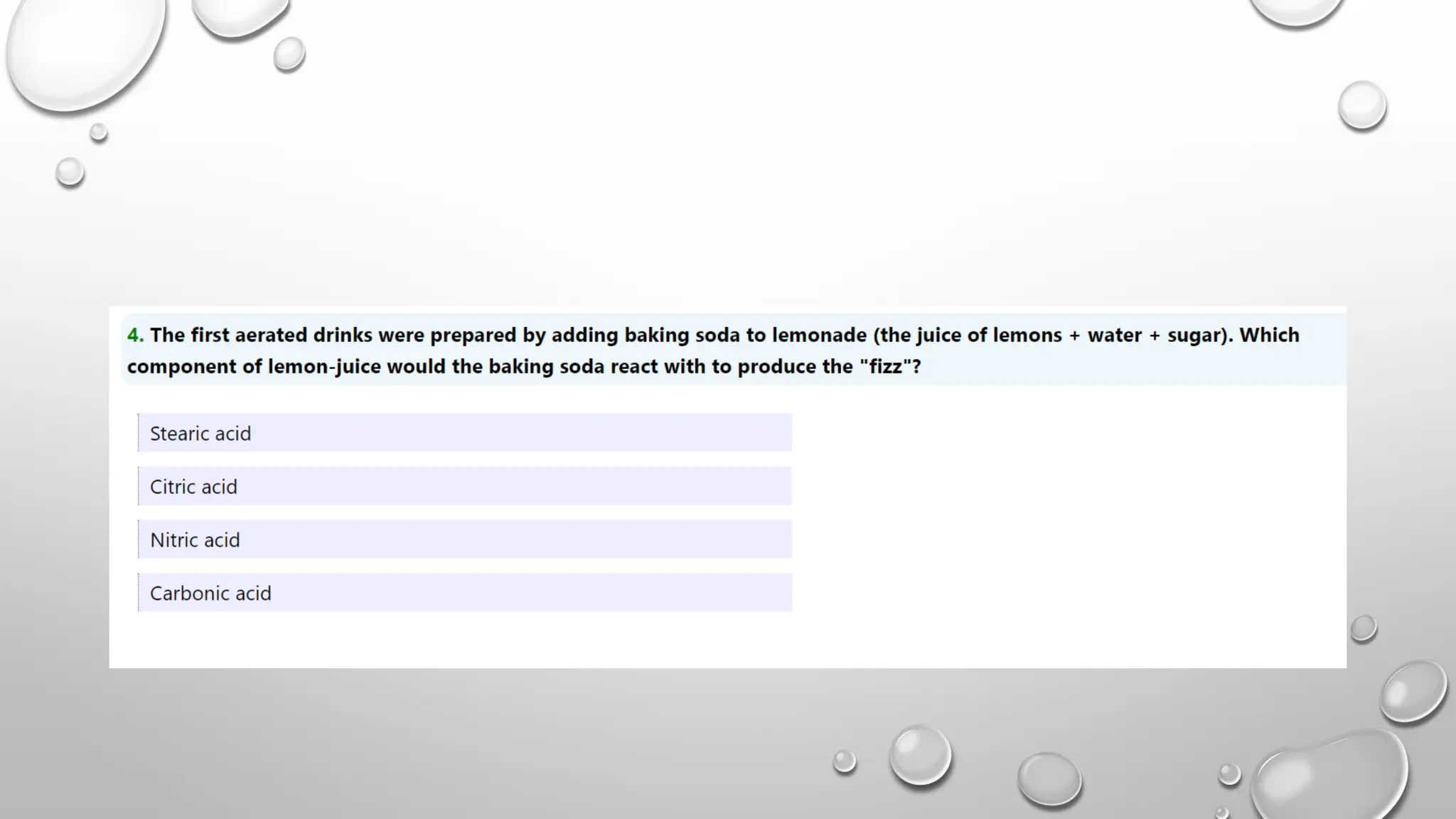
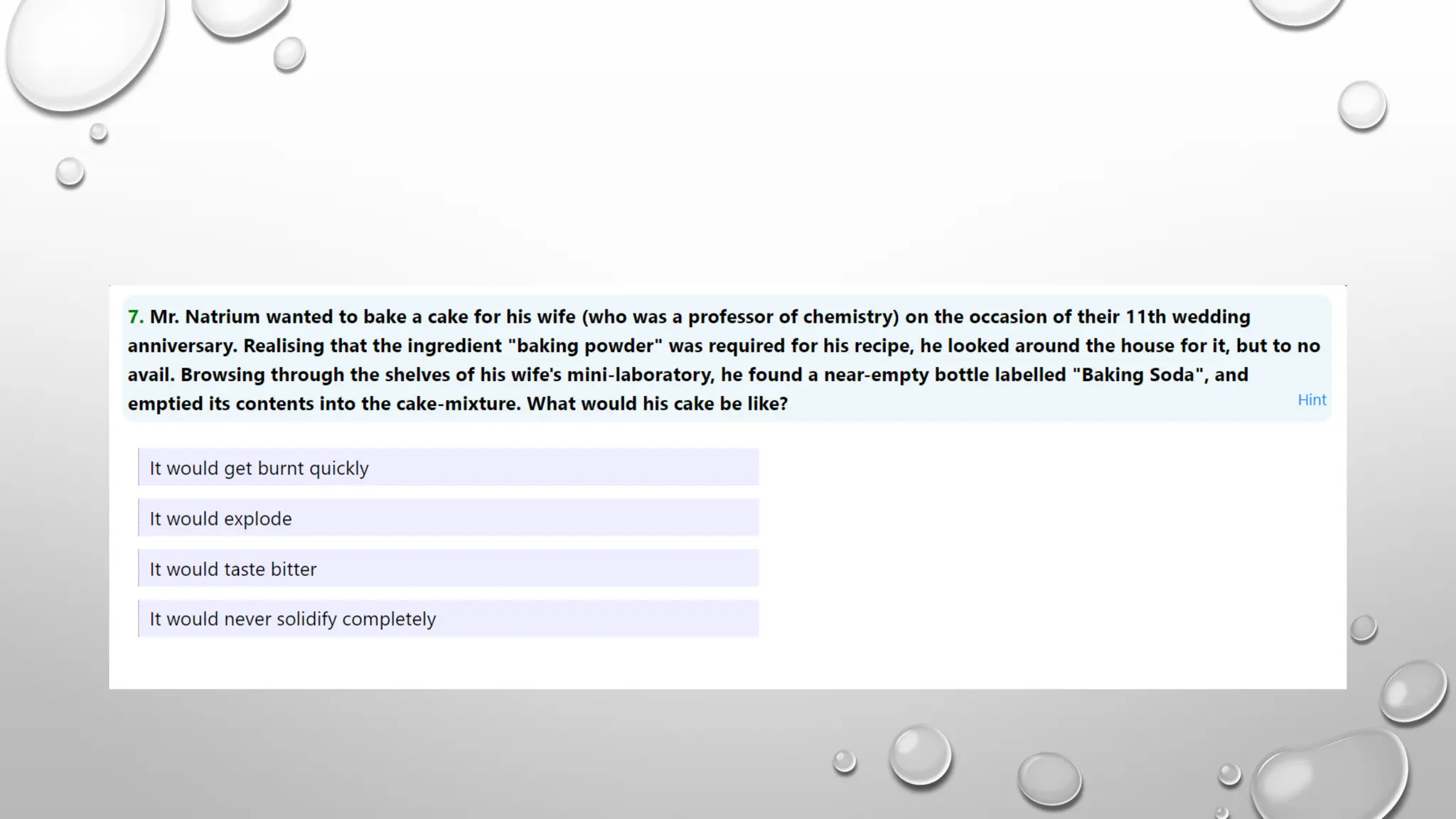
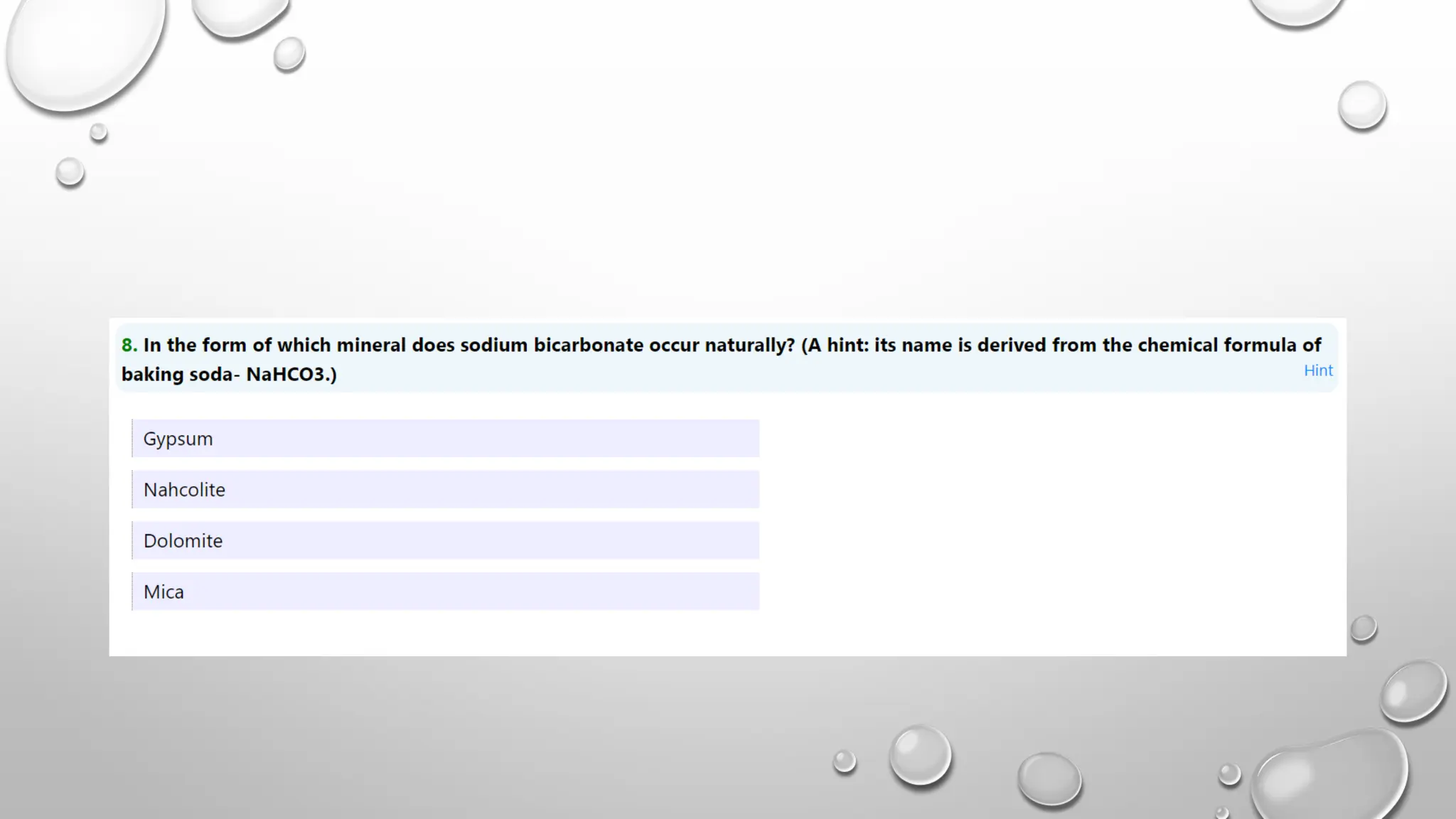
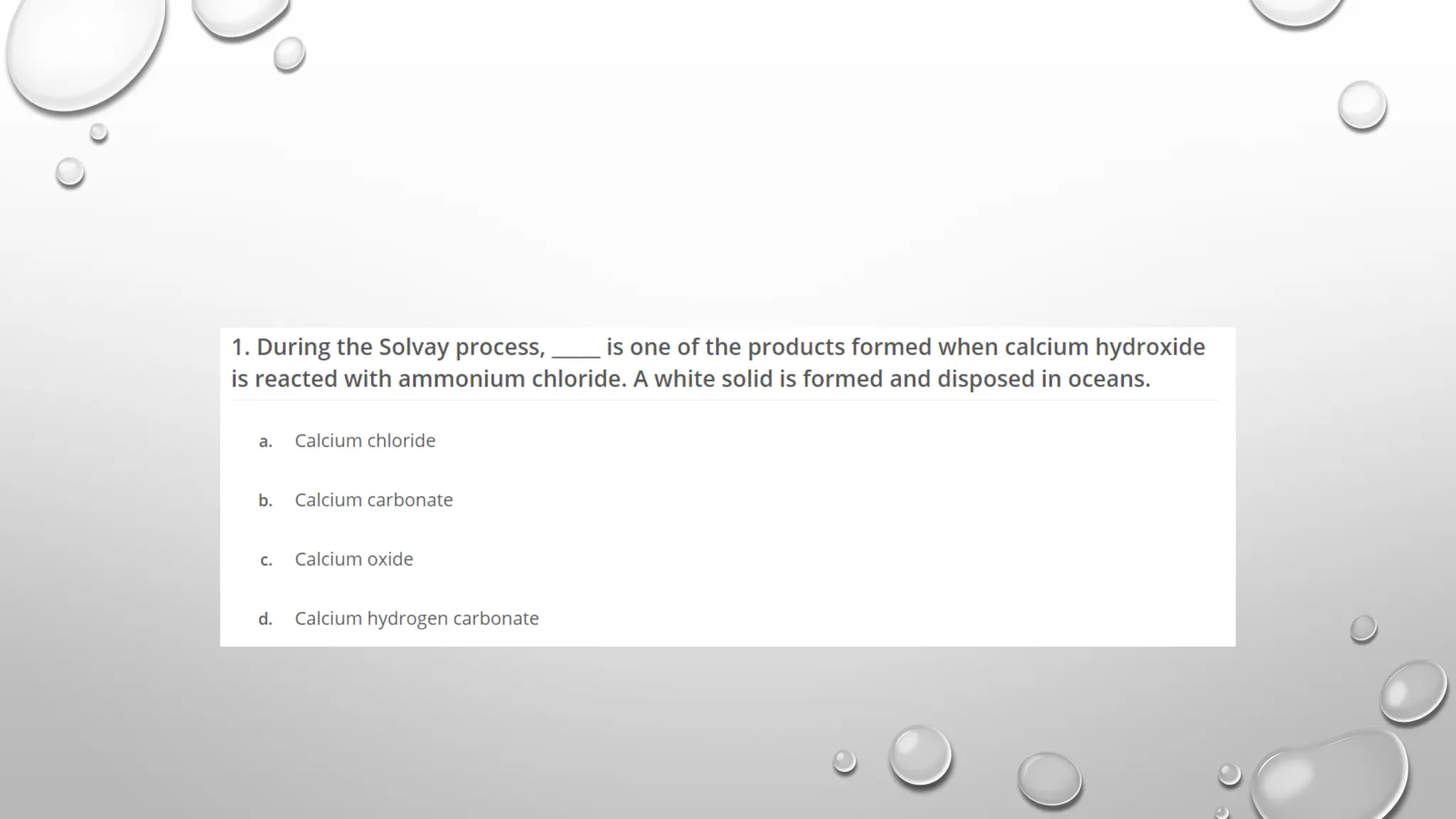
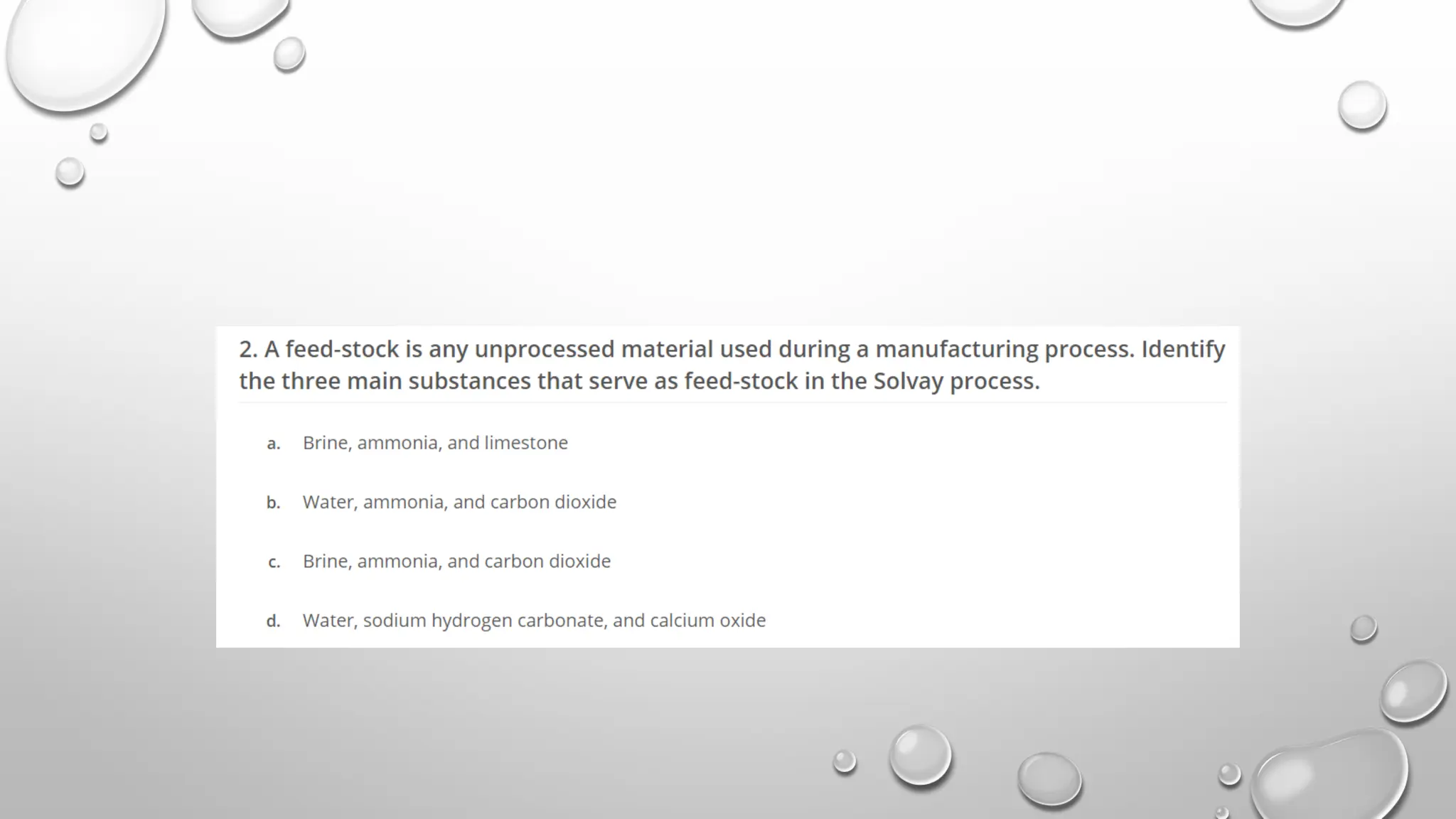
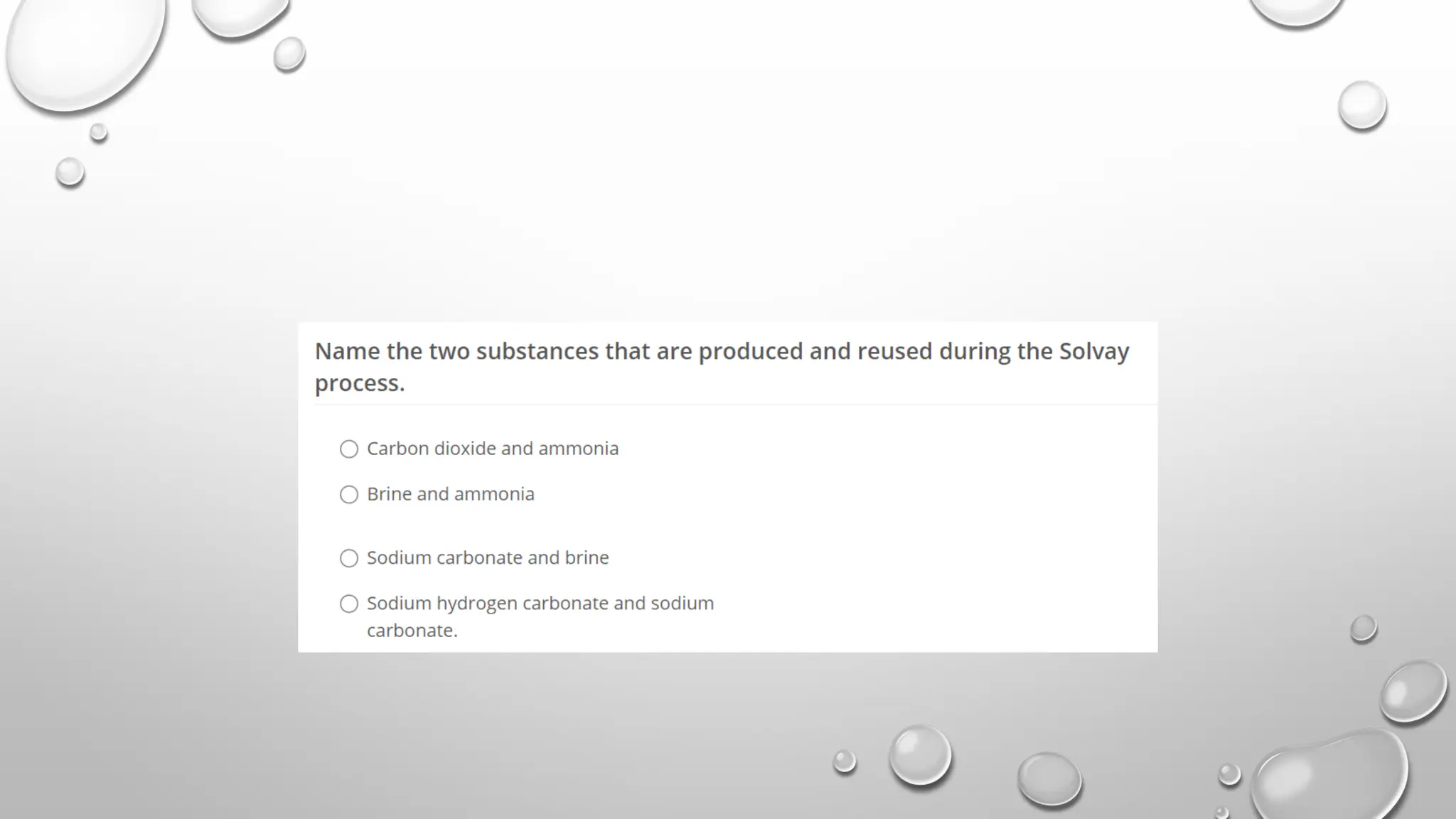
The document discusses the properties, reactions, and uses of sodium chloride (table salt), sodium bicarbonate (baking soda), and sodium carbonate (washing soda). It describes various chemical processes such as electrolysis, the Solvay process for producing sodium carbonate, and methods for separating mixtures. Essential characteristics of these compounds include their physical states, solubility, and applications in daily life and industrial processes.