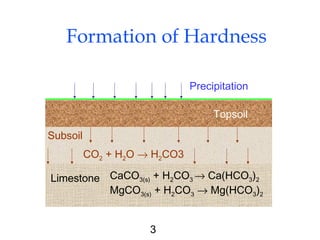
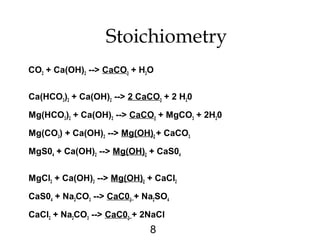
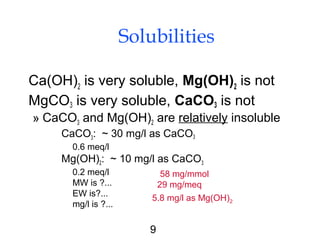
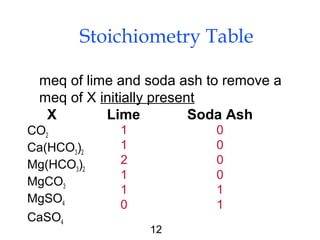
Water softening removes hardness primarily caused by calcium and magnesium ions through precipitation or ion exchange processes. Hardness above 150 mg/L as CaCO3 can cause issues like increased soap consumption and scaling. Hardness is formed as carbon dioxide interacts with limestone and other minerals in soil and groundwater. There are two types of hardness - carbonate (temporary) and non-carbonate (permanent) - which are removed through different softening methods. Lime-soda ash softening uses the addition of lime and soda ash to precipitate calcium and magnesium out of solution, leaving softer water. This process can typically reduce hardness to 80-100 mg/L as CaCO3.