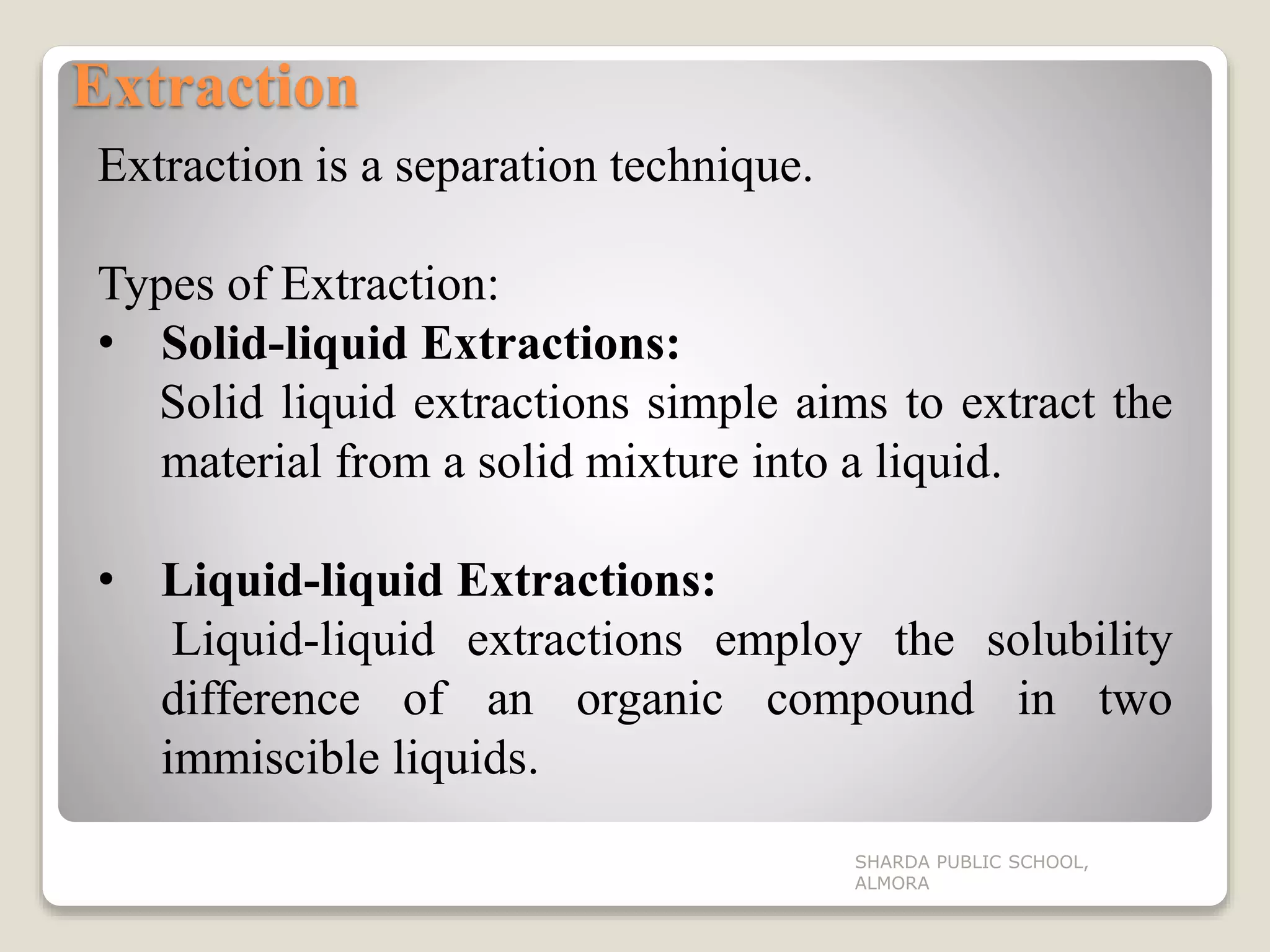
The document discusses the physical properties of organic compounds, focusing on melting point, boiling point, density, and solubility, along with their measurement methods and influencing factors. It also outlines various separation and purification techniques such as extraction, filtration, distillation, and crystallization. Understanding these properties and techniques is essential for applications in chemistry and pharmaceutical science.



















![pH
Henderson-Hasselbach equation:
pH = pKa + log ([A-] / [HA])
•pH = pka; the concentration of ionized and non-ionized forms are
equal
•pH > pKa---the concentration of ionized >non-ionized forms
•pH < pKa---the concentration of ionized <non-ionized forms
Carboxylic acid ---pKa=4-5
HA, PH=7 more than 99 % A-
If an organic molecule is insoluble in a liquid, it precipitates.
precipitation and dissolution might be evaluated as opposite actionsSHARDA PUBLIC SCHOOL,
ALMORA](https://image.slidesharecdn.com/phsical-props-and-measurement-160219160204/75/Separation-and-Purification-of-organic-compounds-22-2048.jpg)