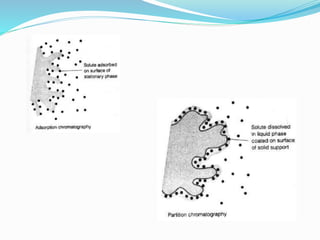
The document provides a comprehensive overview of chromatography, detailing various types such as TLC, HPTLC, and HPLC, along with their principles, applications, and historical development. It explains the separation processes based on physical and chemical properties of stationary and mobile phases, and outlines the advantages and disadvantages of each technique. Additionally, it discusses the components and procedures for executing experiments in thin layer chromatography and high-performance liquid chromatography.