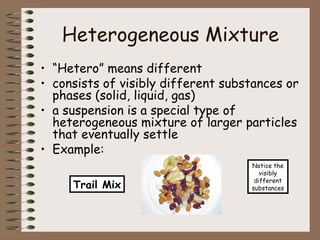
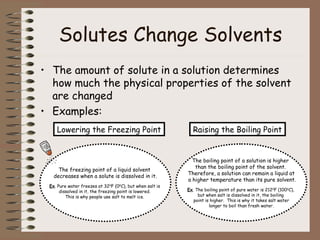
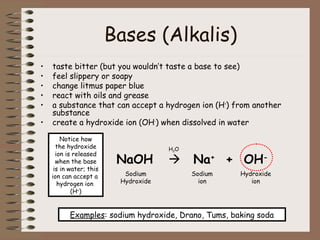
This document discusses different types of mixtures including heterogeneous and homogeneous mixtures. Heterogeneous mixtures are mixtures with visibly different parts that can be separated, like trail mix. Homogeneous mixtures are uniform mixtures like salt water solutions that cannot be easily separated. Solutions are a type of homogeneous mixture where a solute dissolves evenly throughout a solvent. Concentration, solubility, acids, bases, and the pH scale are also explained in the context of mixtures and solutions.