Embed presentation
Downloaded 205 times




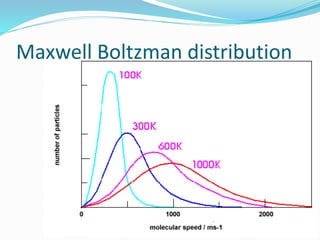
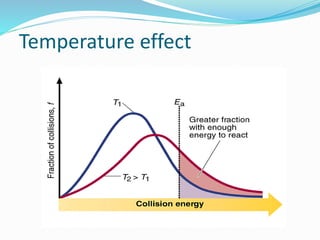
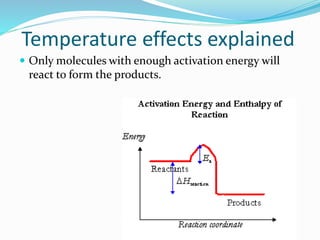
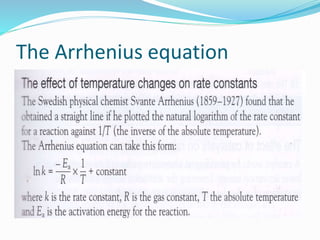
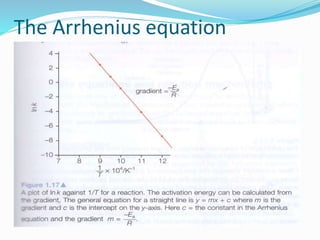

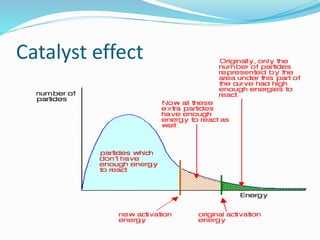
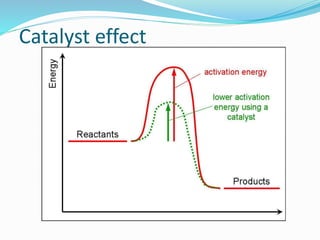
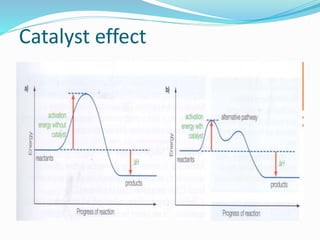
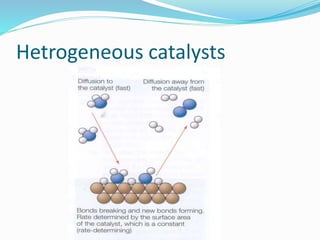
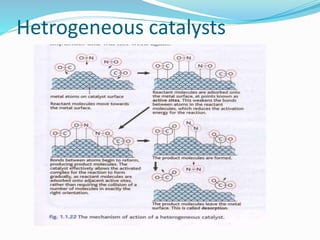
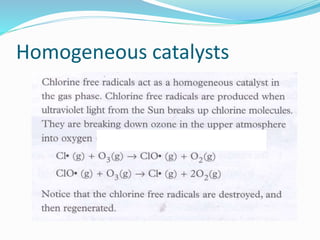
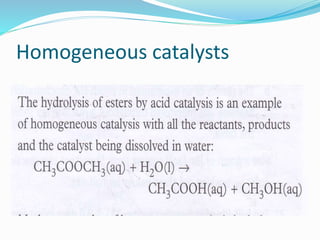
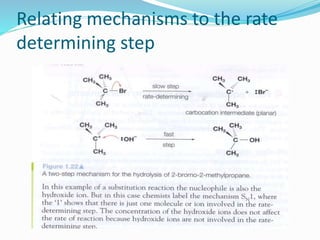
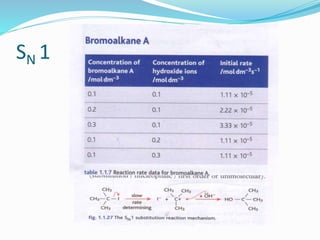
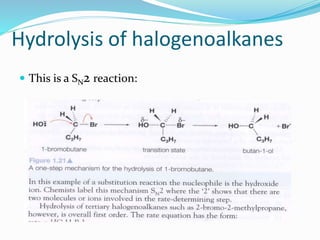
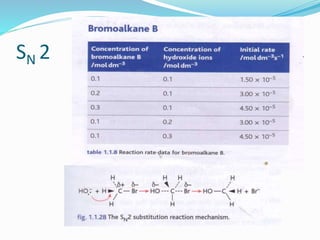
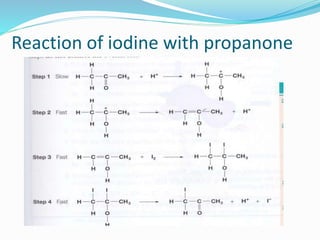
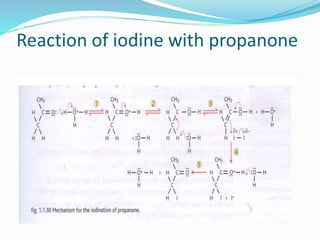

The document discusses several factors that affect the rate of chemical reactions: 1) Concentration and surface area - Increasing concentration and surface area increases the number and frequency of collisions between reacting particles, speeding up reactions. 2) Temperature - Higher temperatures cause particles to collide more energetically, increasing reaction rates. A 10 degree rise often doubles the rate. More particles have energy exceeding the activation energy at higher temperatures. 3) Catalysts - Catalysts increase reaction rates by lowering the activation energy needed, allowing reactions to proceed more quickly without being consumed in the process.






















