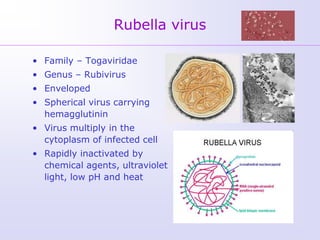
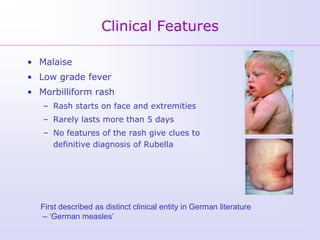
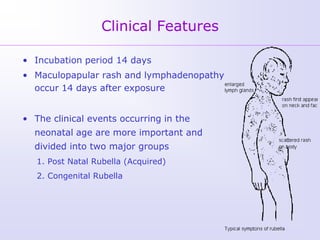
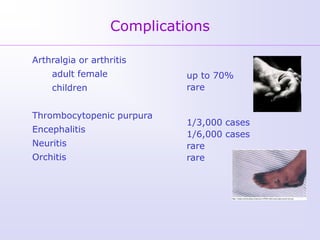
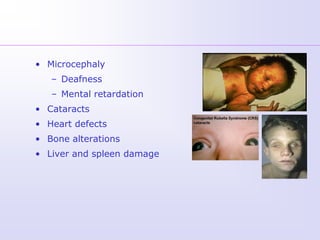
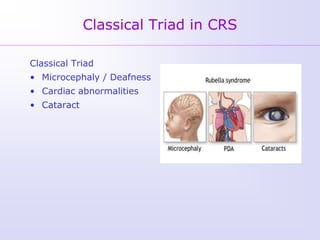
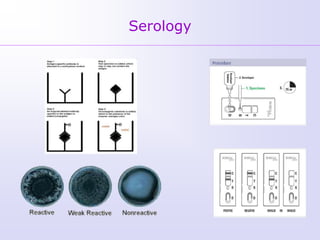
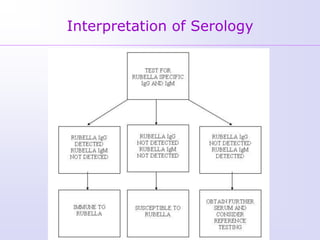
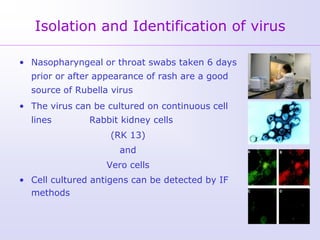
1) Rubella, also known as German measles, is a mild viral illness caused by the rubella virus. It is transmitted through airborne droplets from coughing or sneezing. While usually mild, infection during pregnancy can cause congenital rubella syndrome in the fetus.
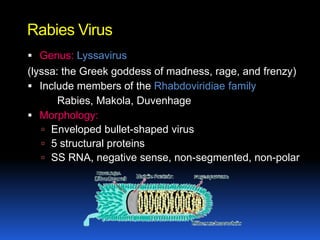
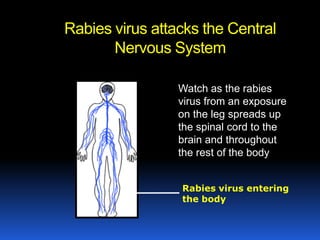
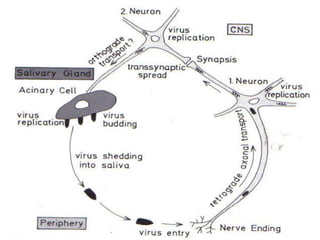
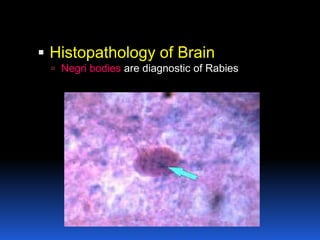
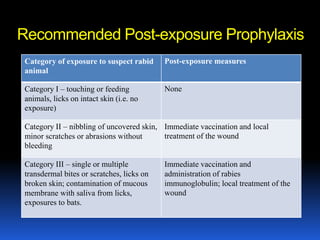
2) Rabies is a fatal viral disease that affects mammals. It is transmitted through bites or scratches from an infected animal. Once symptoms appear, rabies is nearly always fatal. Dogs are the most common source of human rabies infections worldwide.
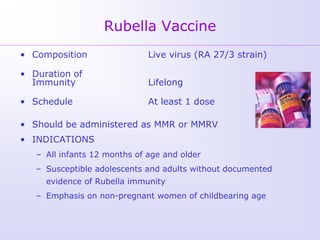
3) Both diseases can be prevented through vaccination. For rubella, vaccination is recommended for all children and non-pregnant women of childbearing age. Post-exposure prophyl