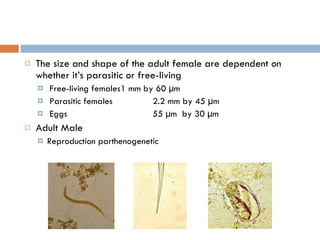
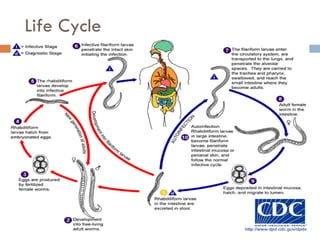
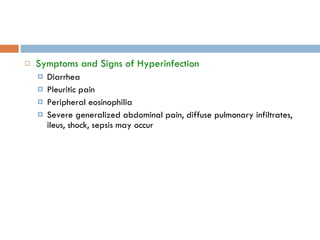
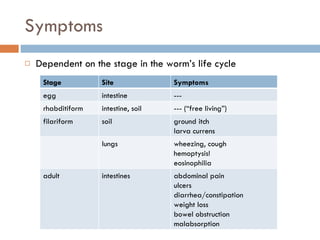
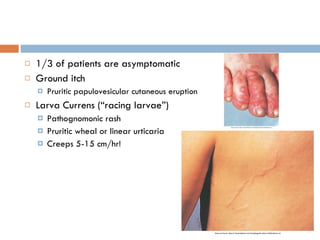
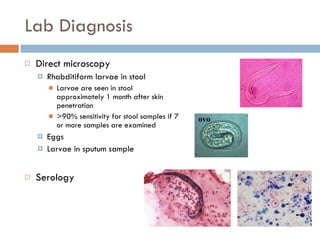
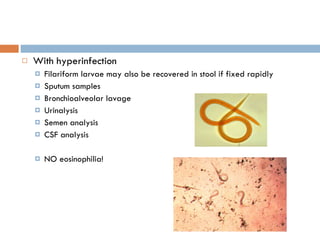
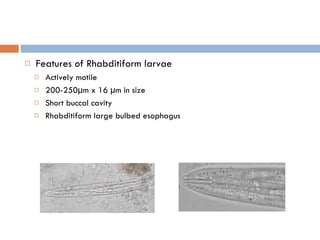
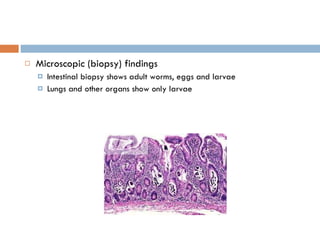
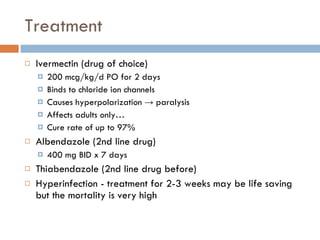
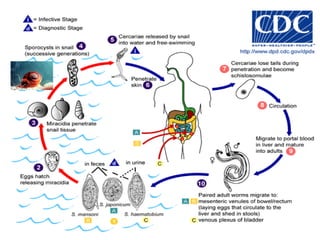
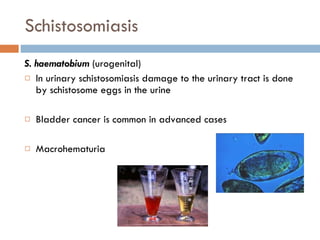
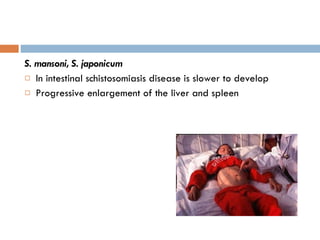
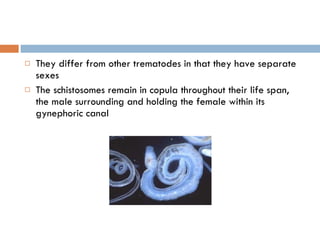
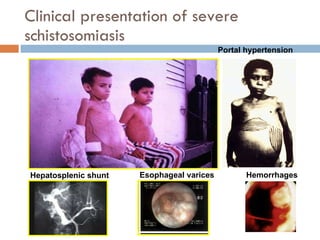
This document summarizes information about two parasitic worms: Strongyloides stercoralis and Schistosoma species. Strongyloides stercoralis can cause strongyloidiasis and potentially fatal hyperinfection in immunocompromised individuals. It has a direct life cycle within the human host. Schistosoma species cause schistosomiasis (bilharzia) which is transmitted through contaminated water and affects over 200 million people globally. The three main species that infect humans reside in blood vessels and cause disease via egg-induced damage. Diagnosis involves detecting eggs in stool/urine and treatment is with praziquantel or oxamniquine.