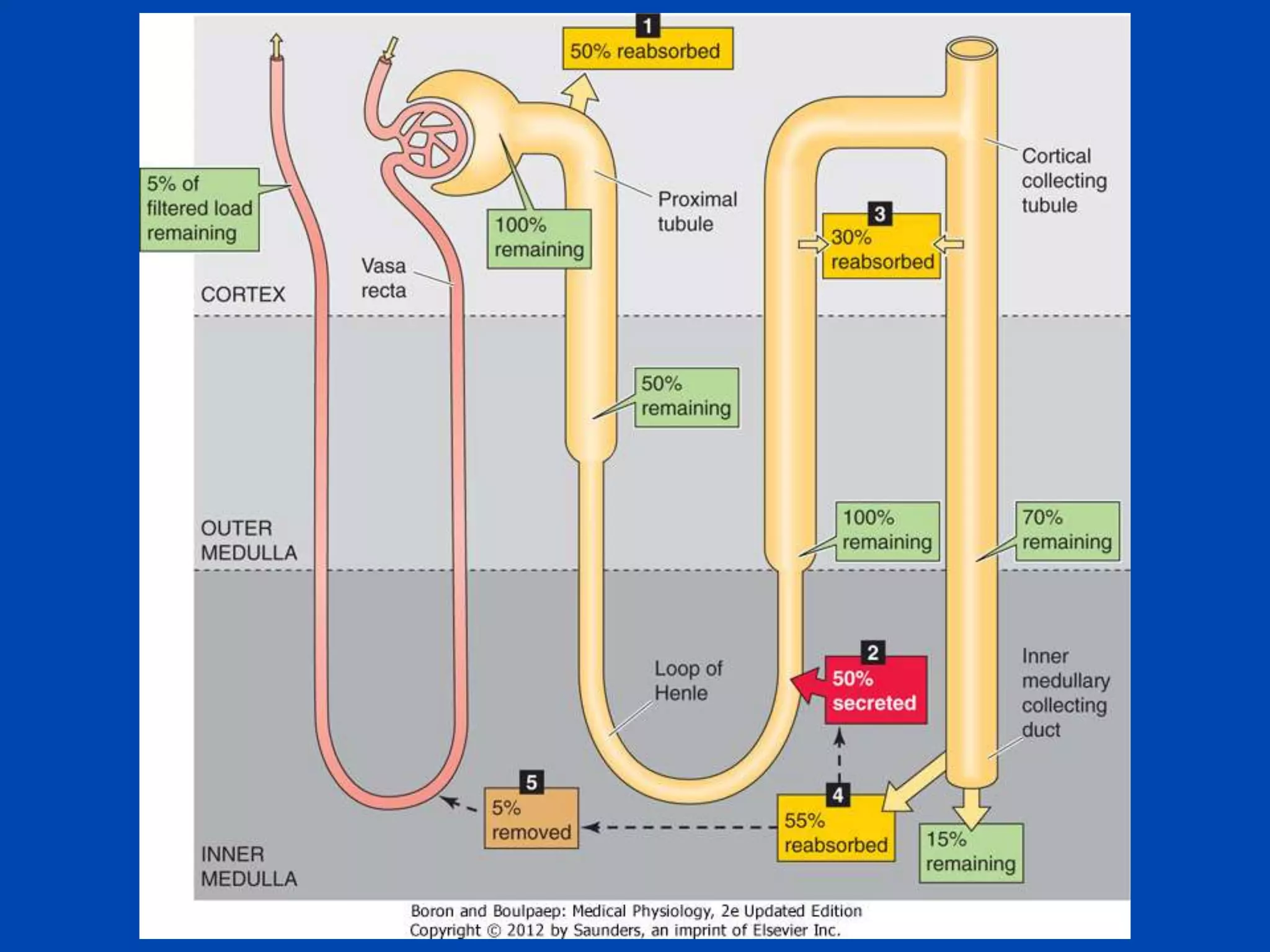
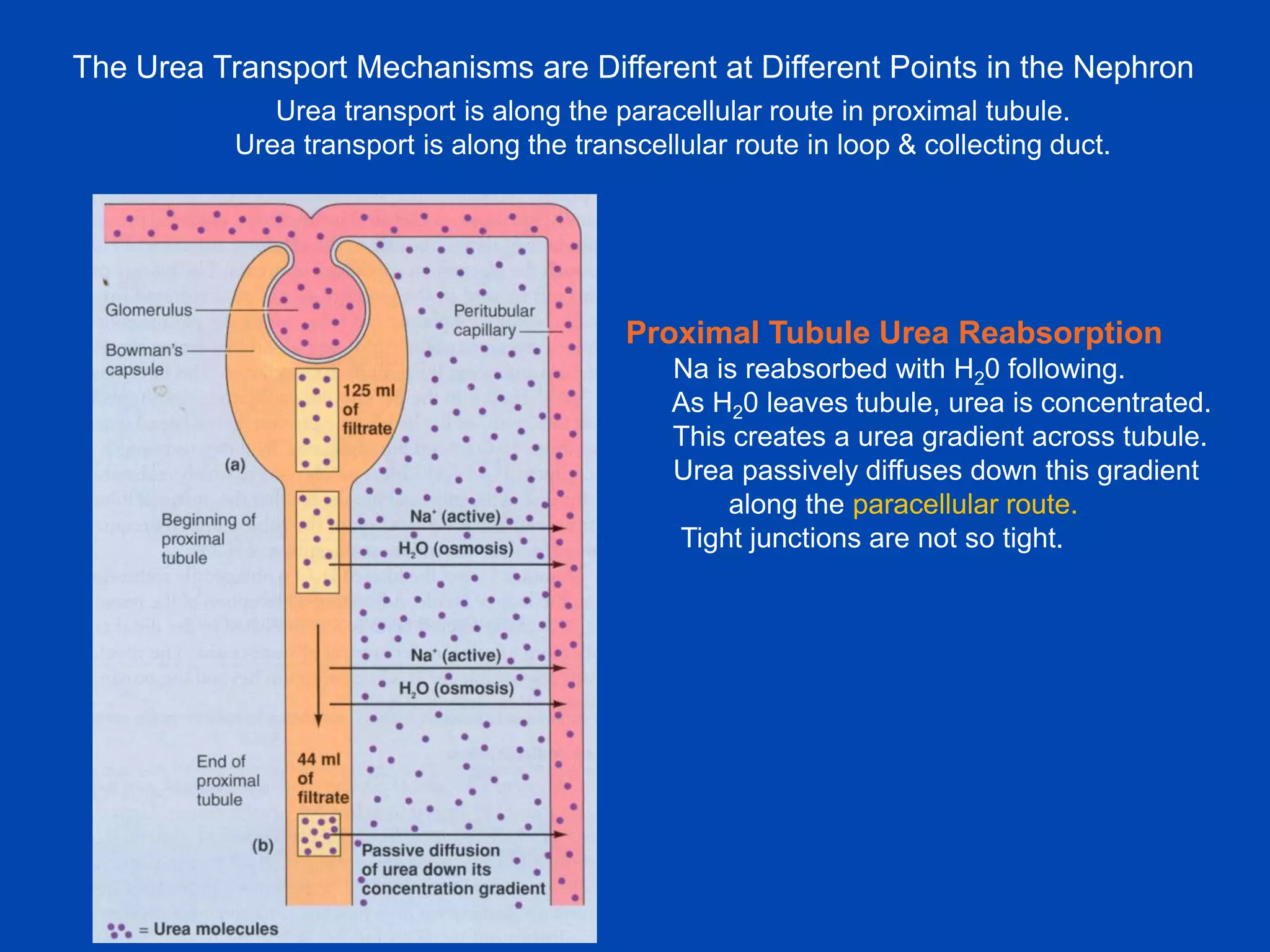
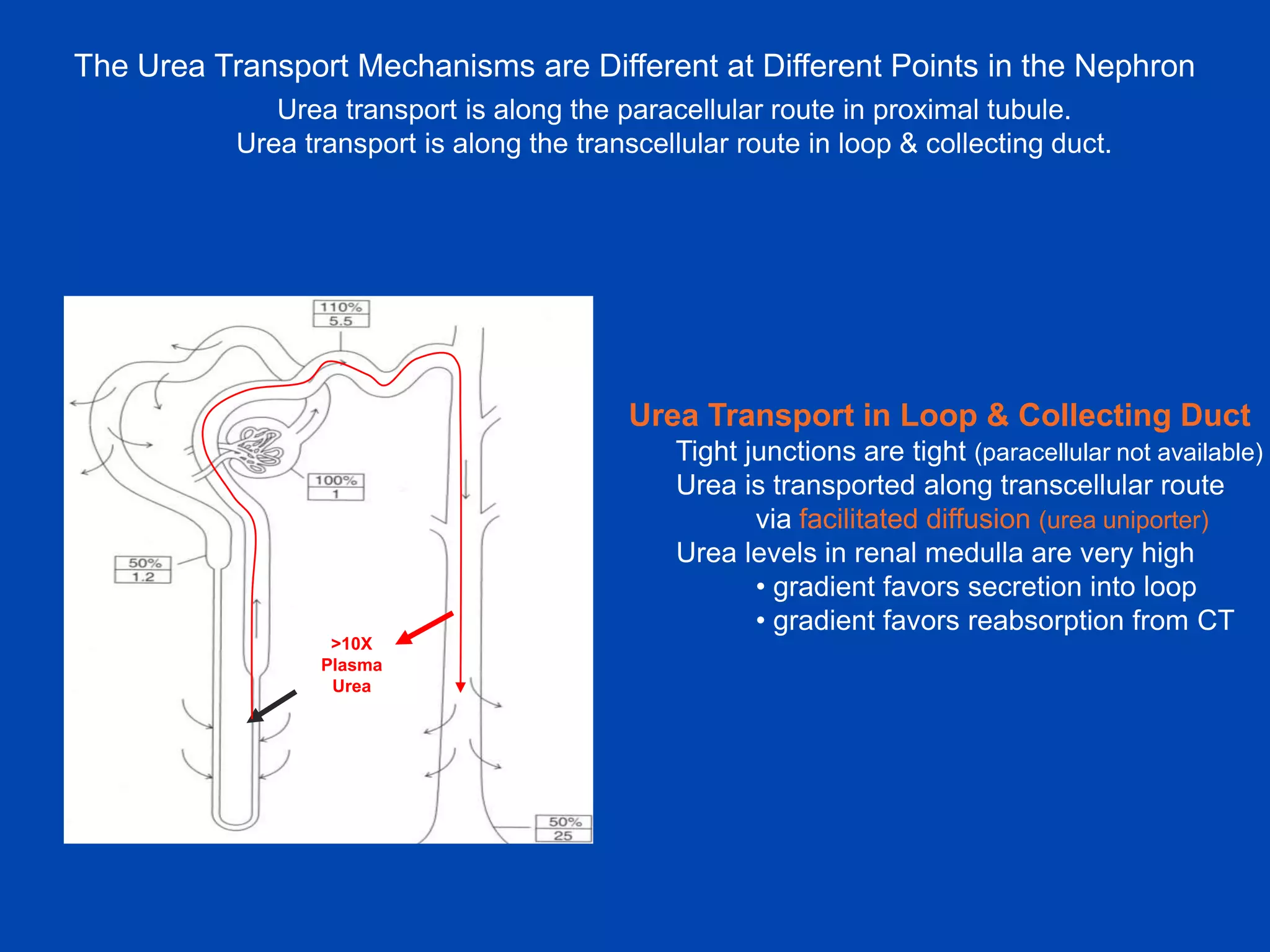
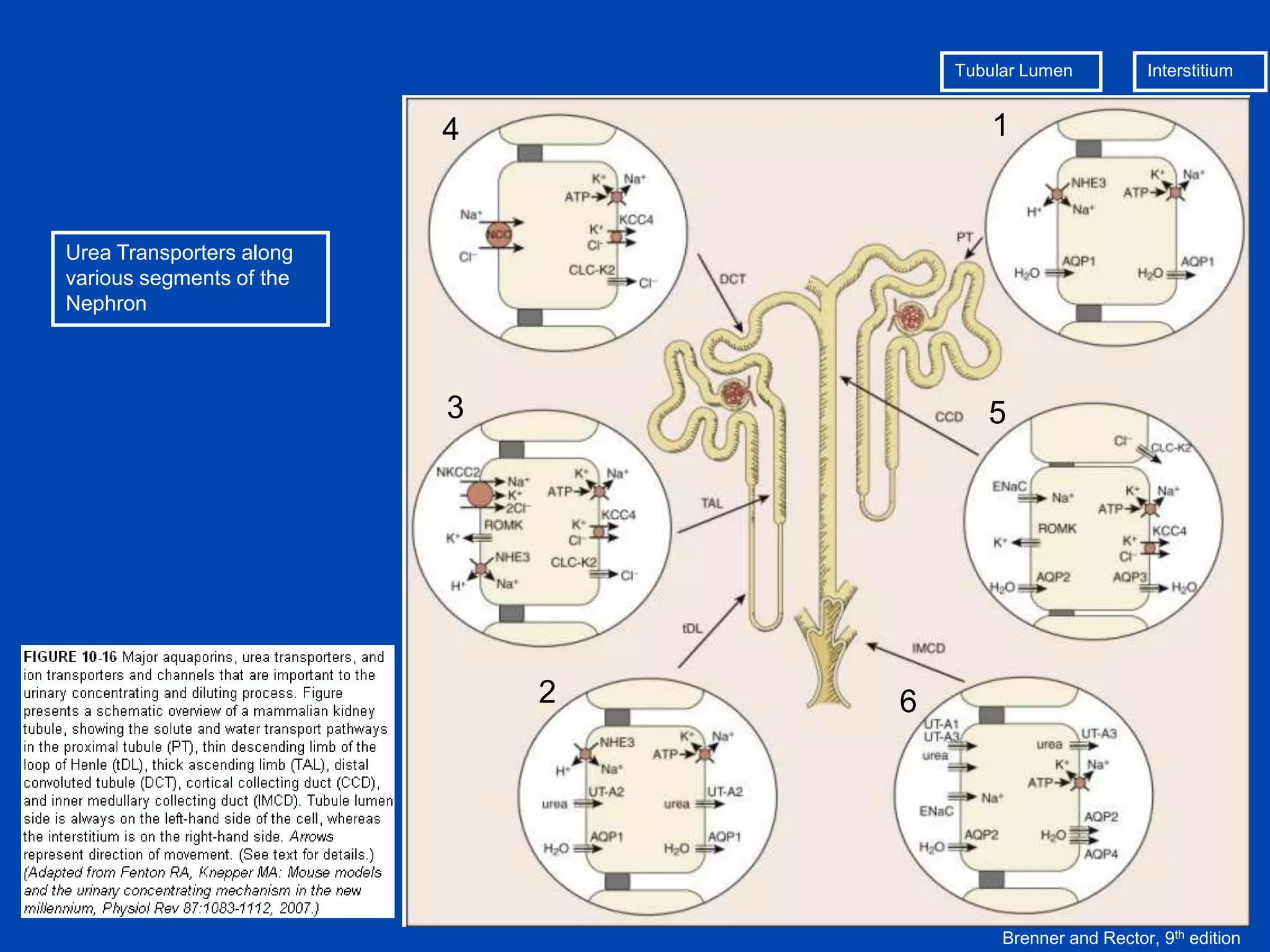
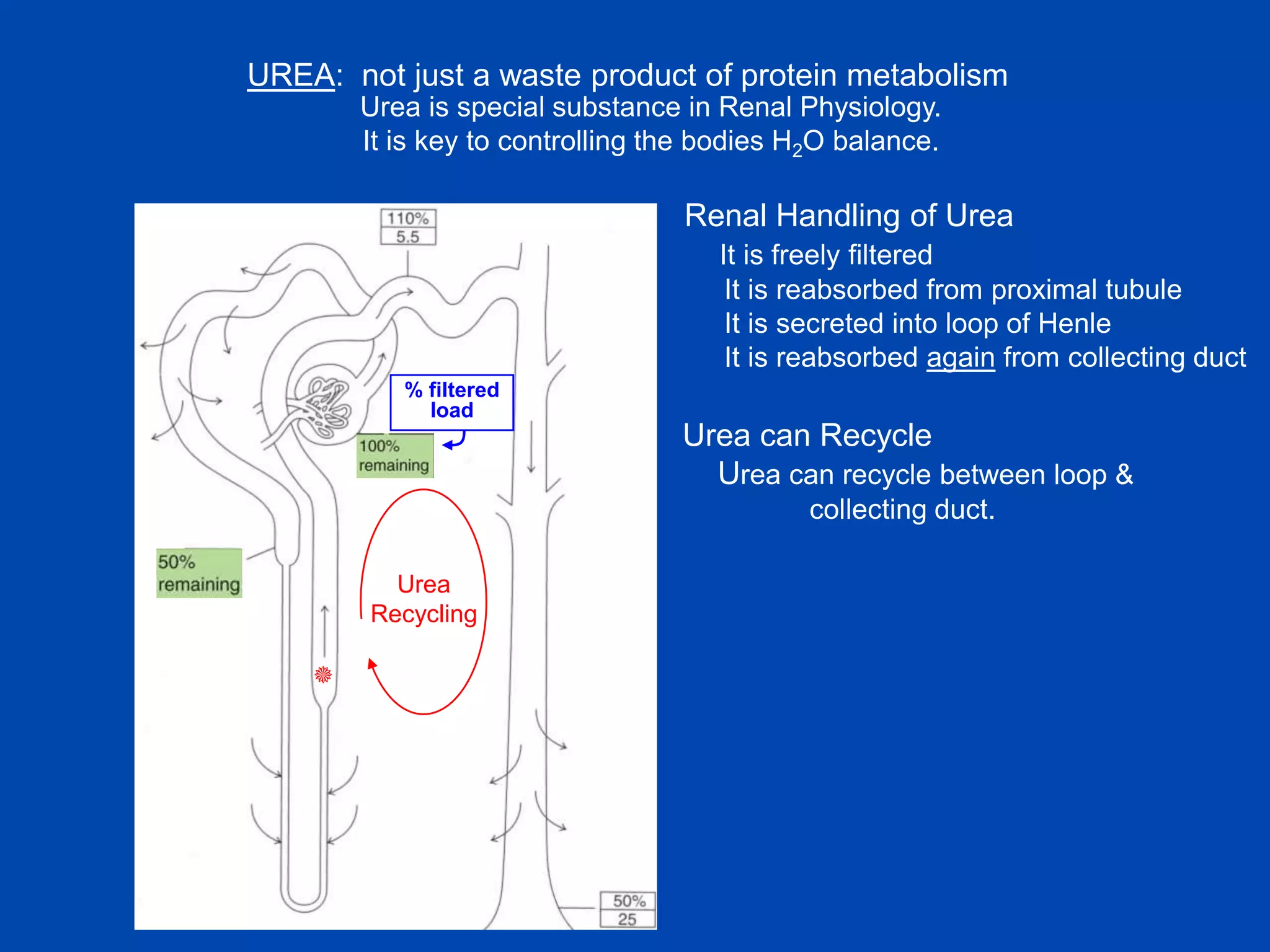
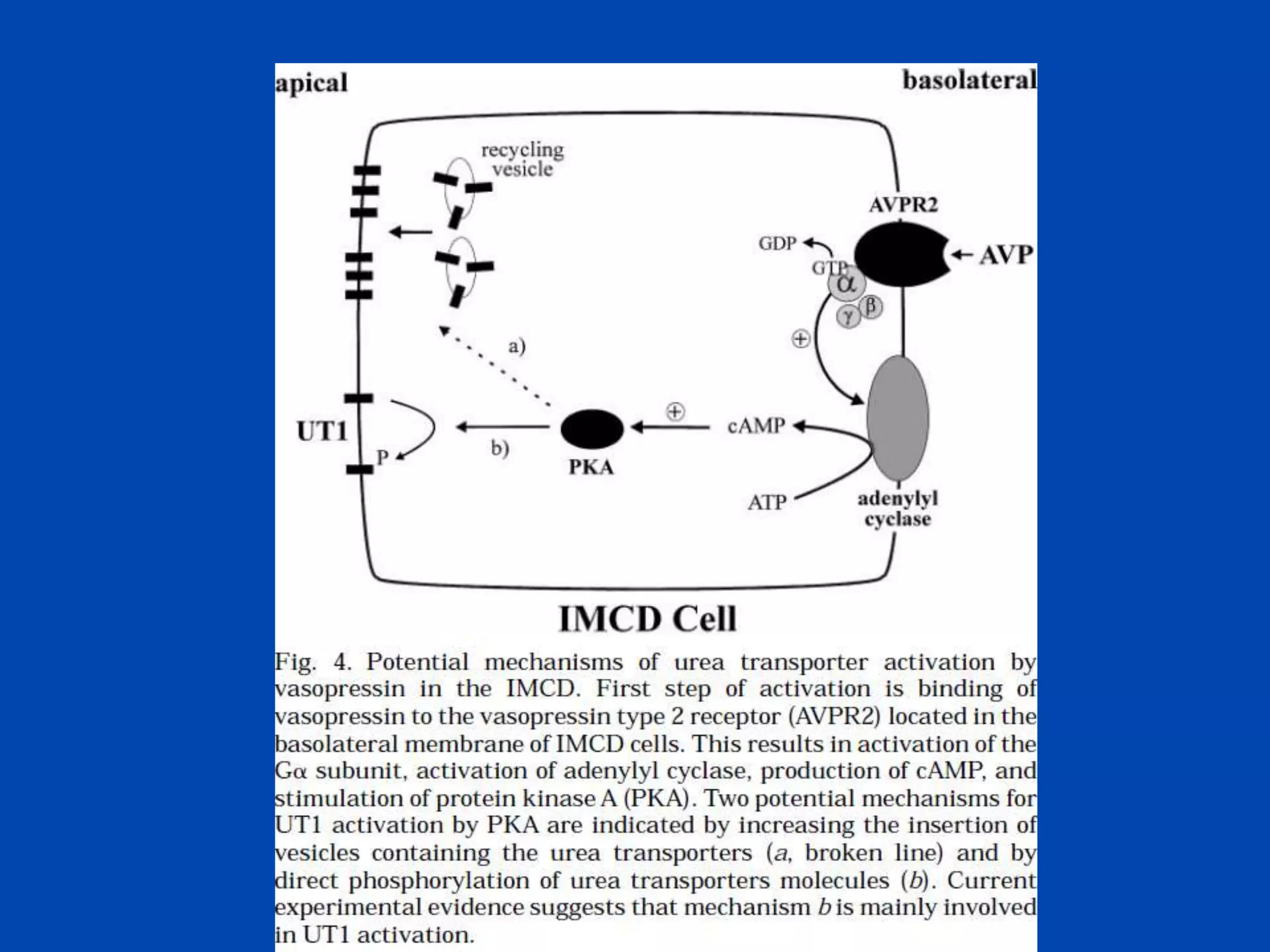
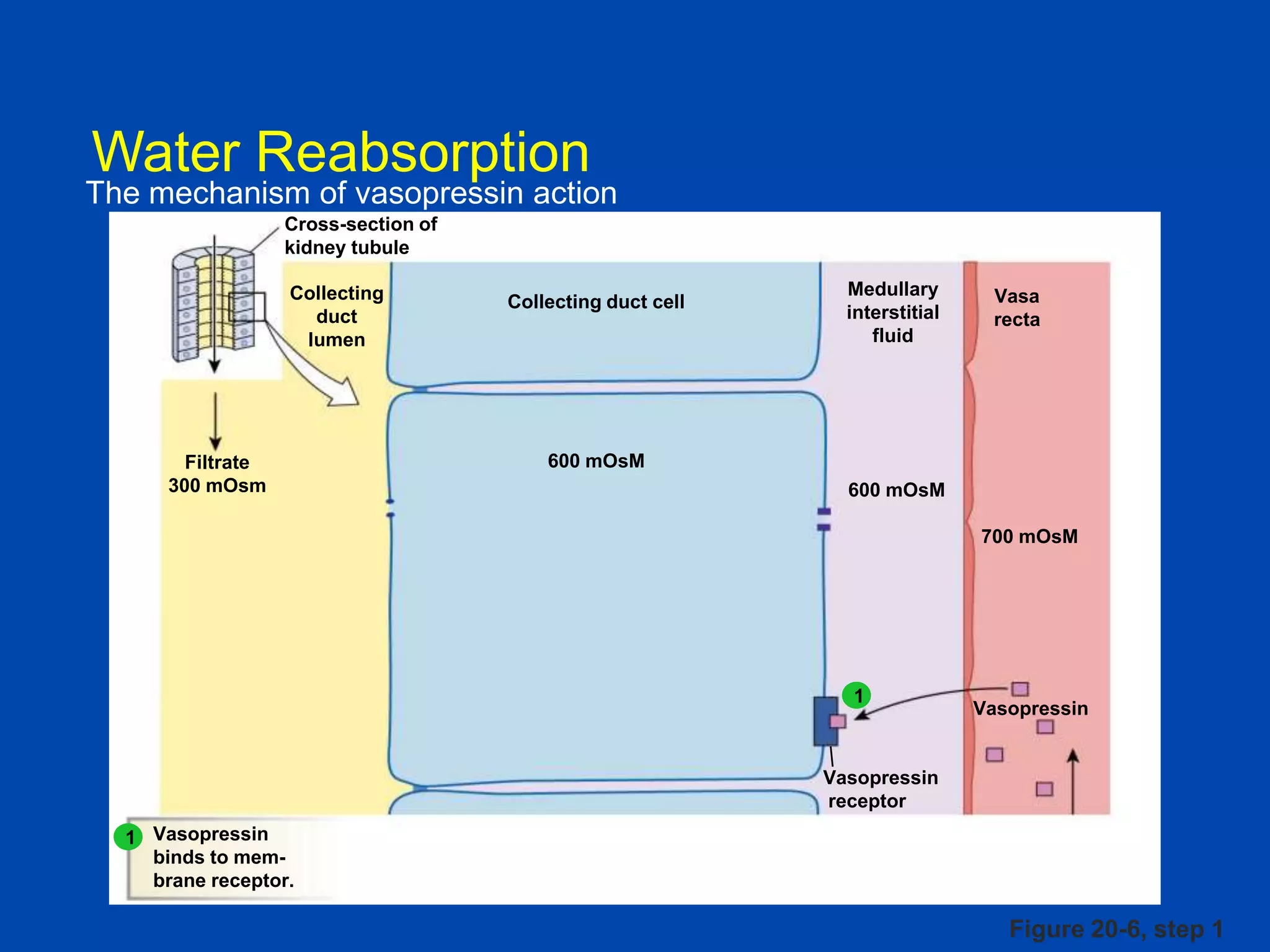
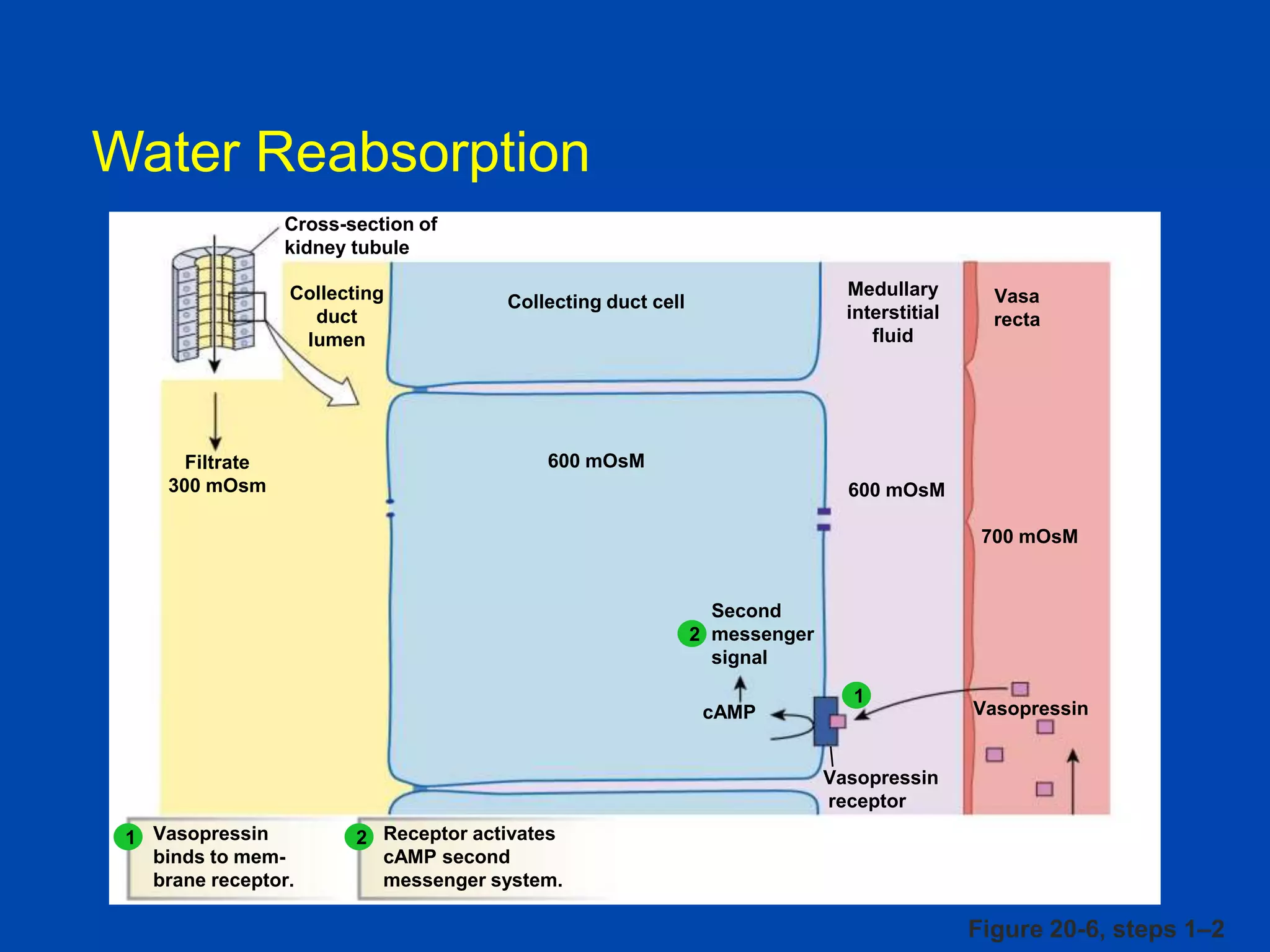
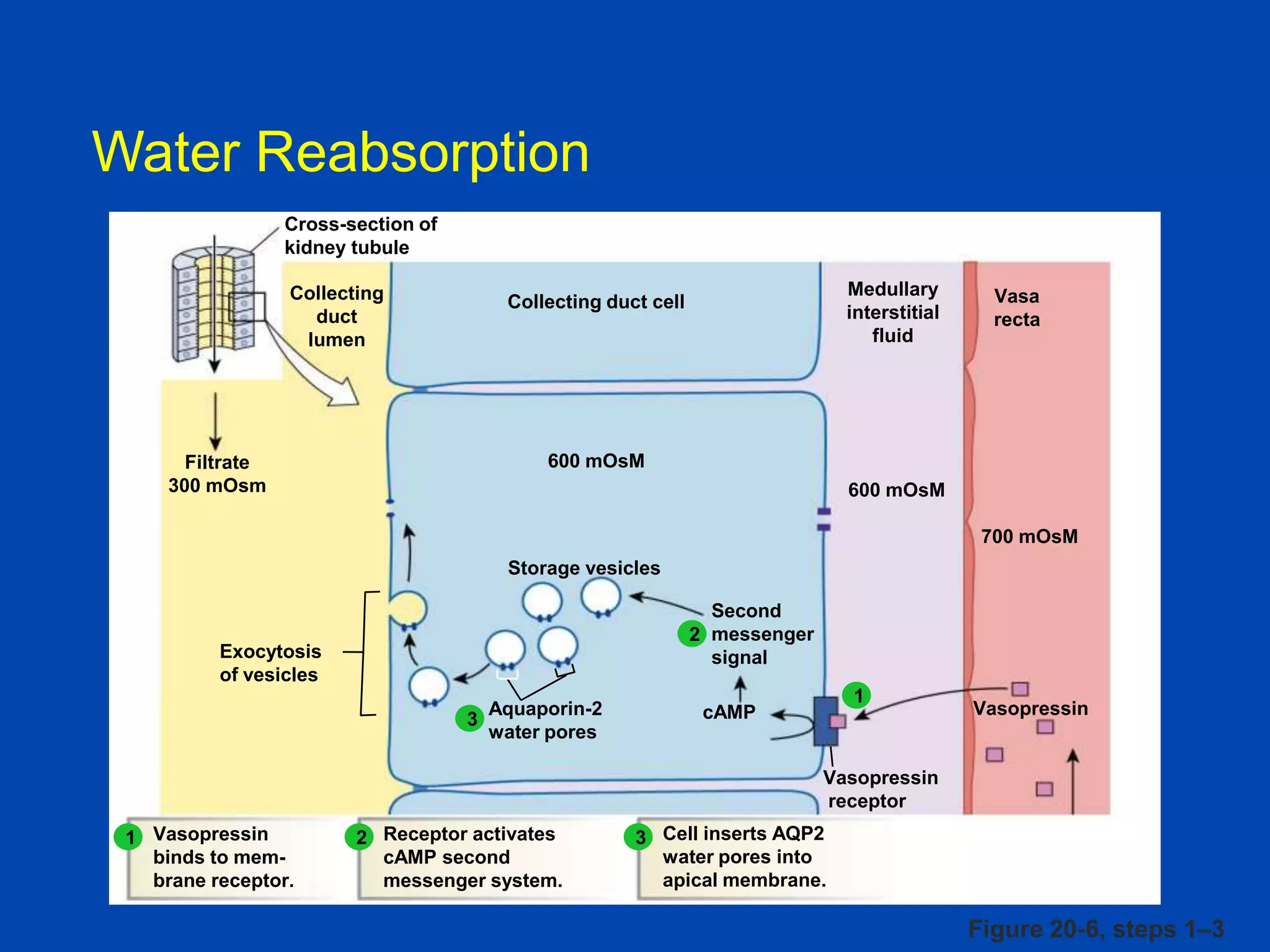
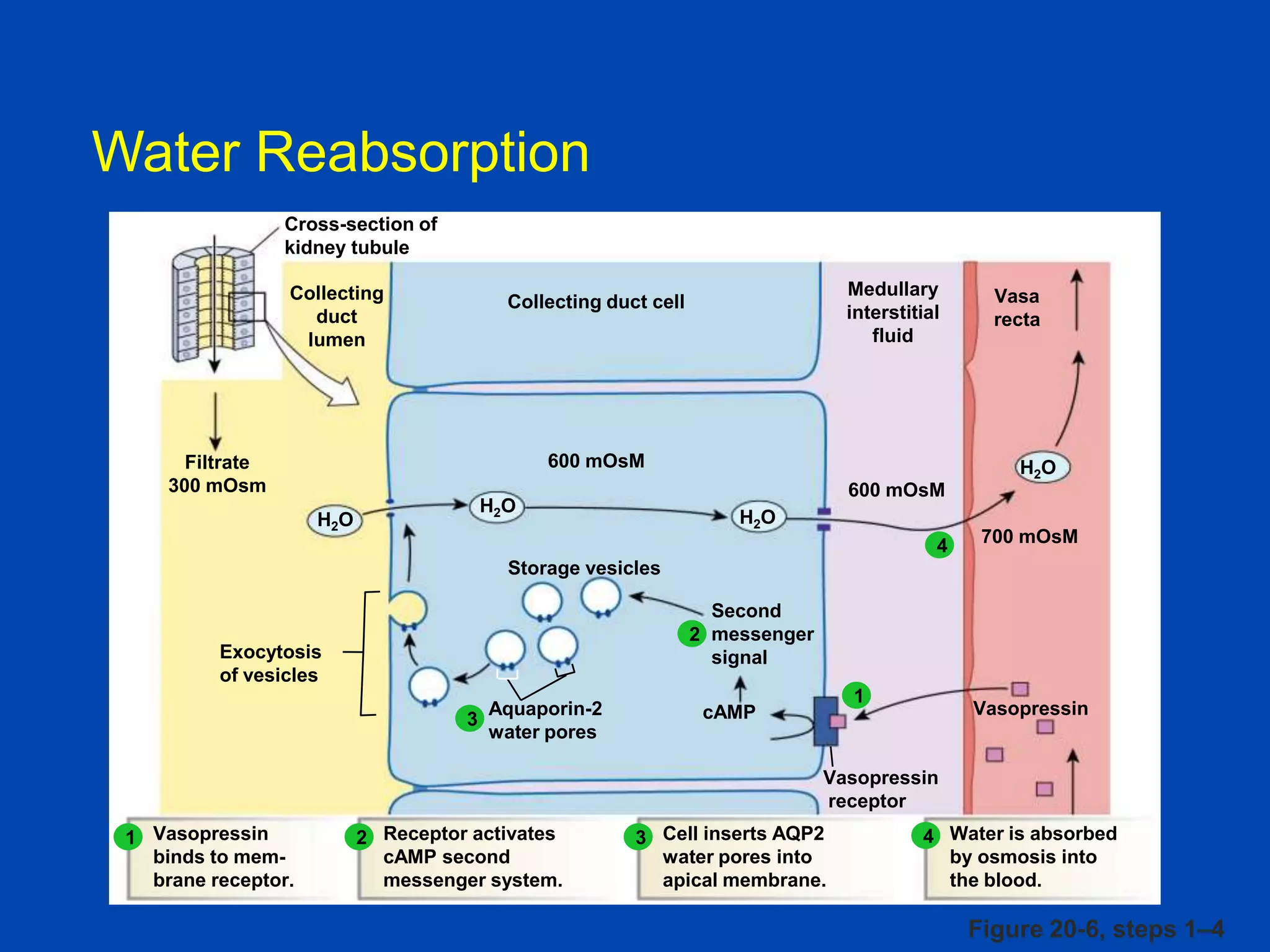
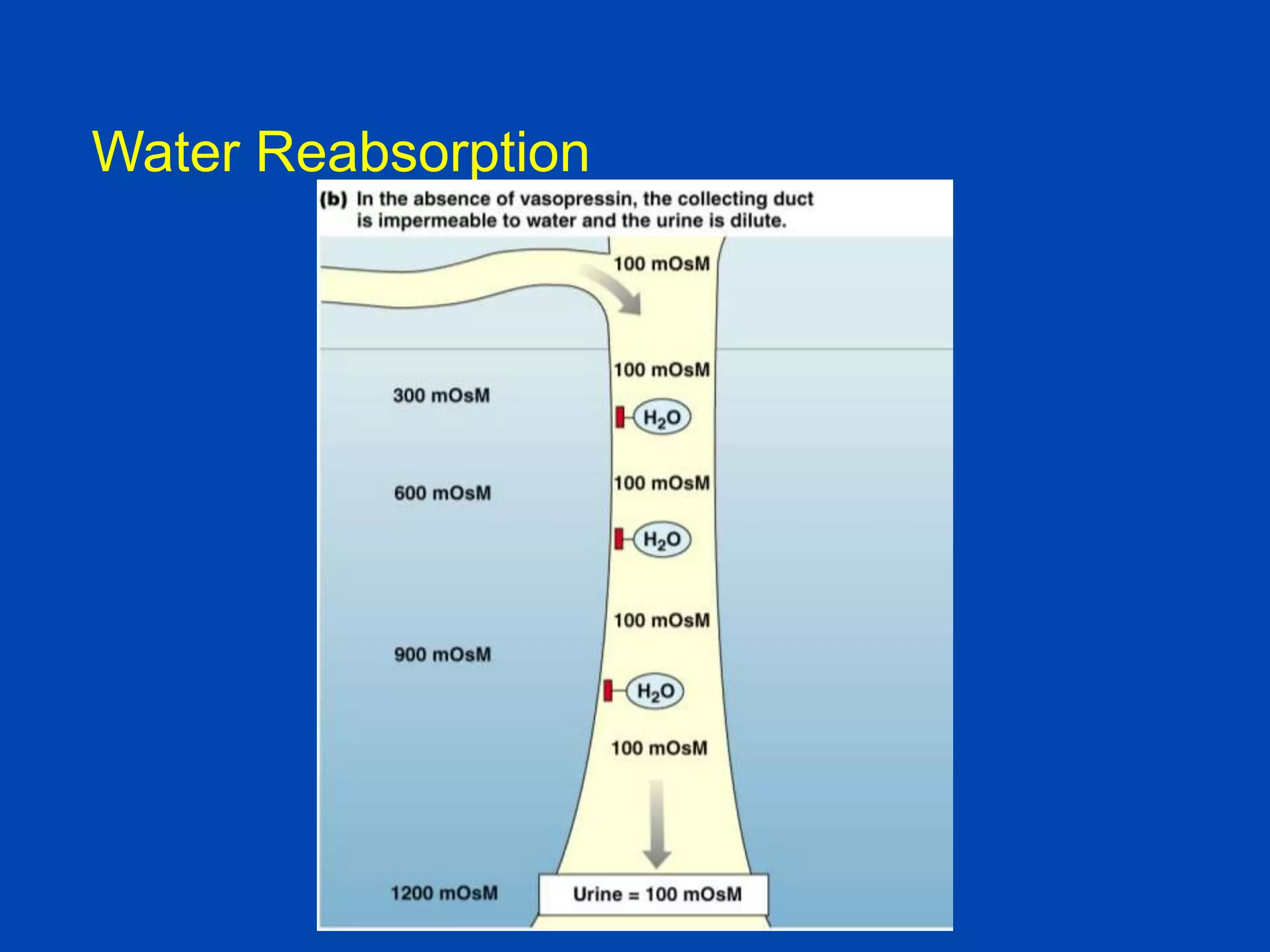
kidney tubule
Collecting
duct
lumen
Filtrate
300 mOsm
Medullary
interstitial
fluid
Collecting duct cell
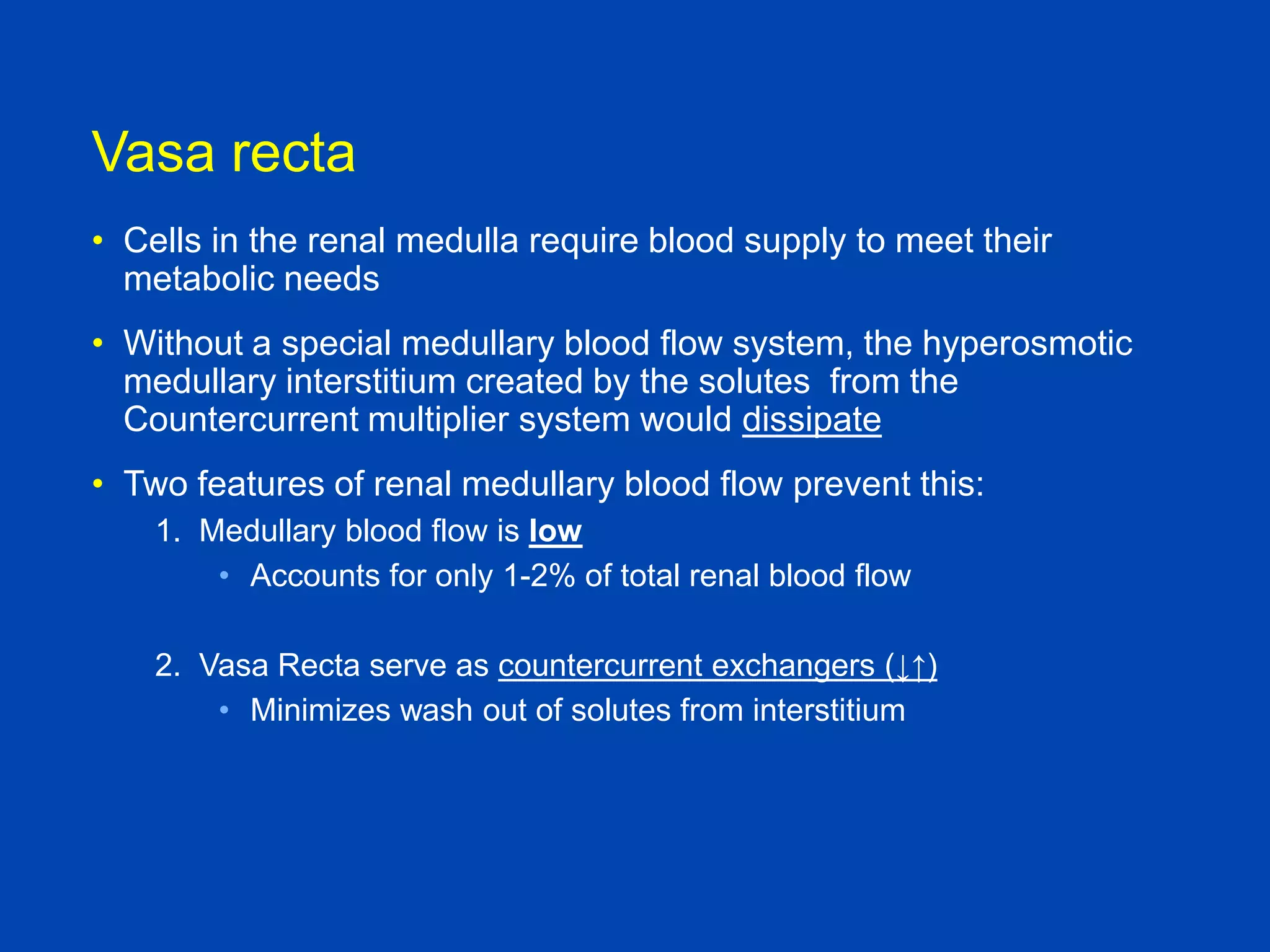
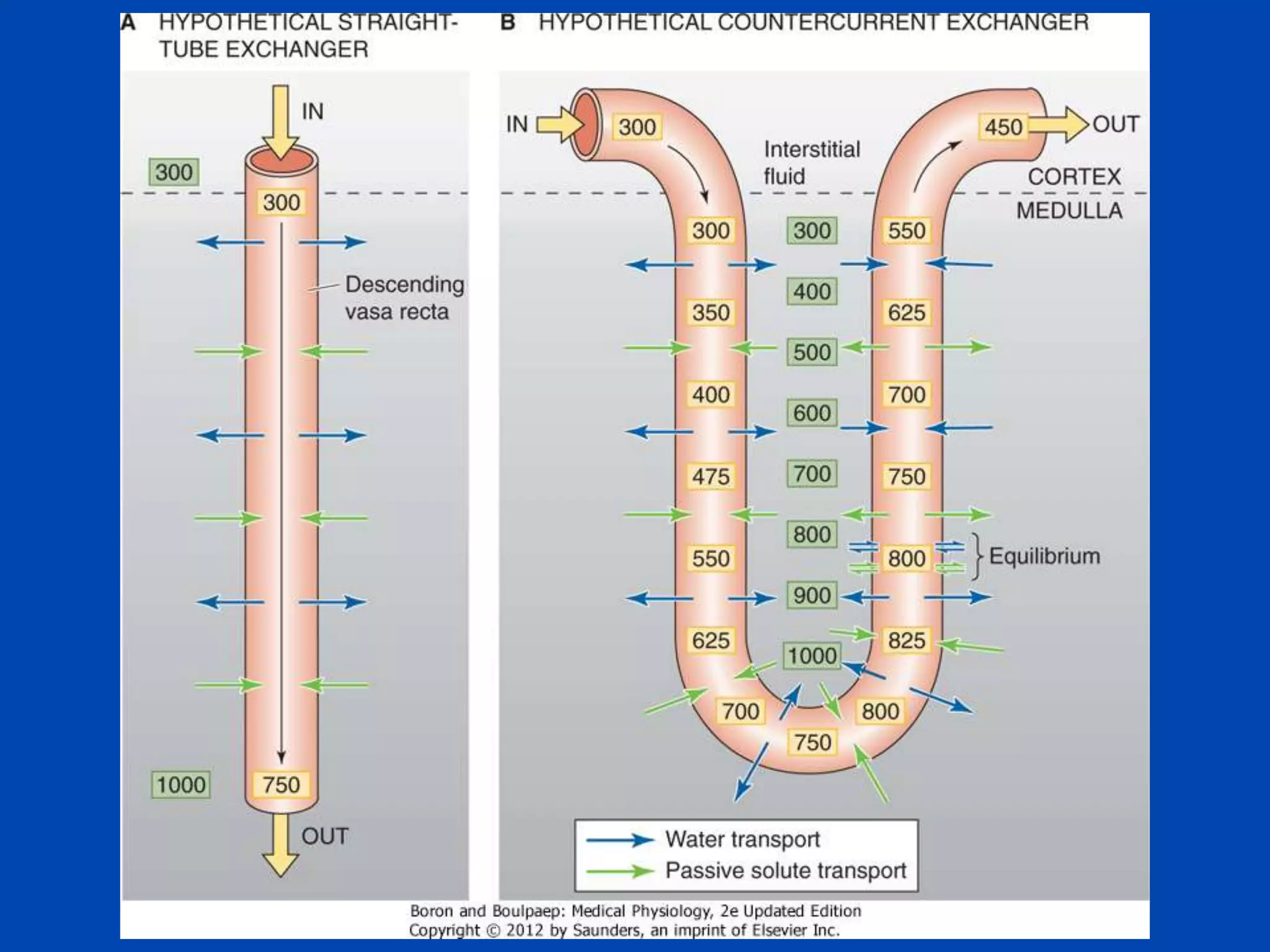
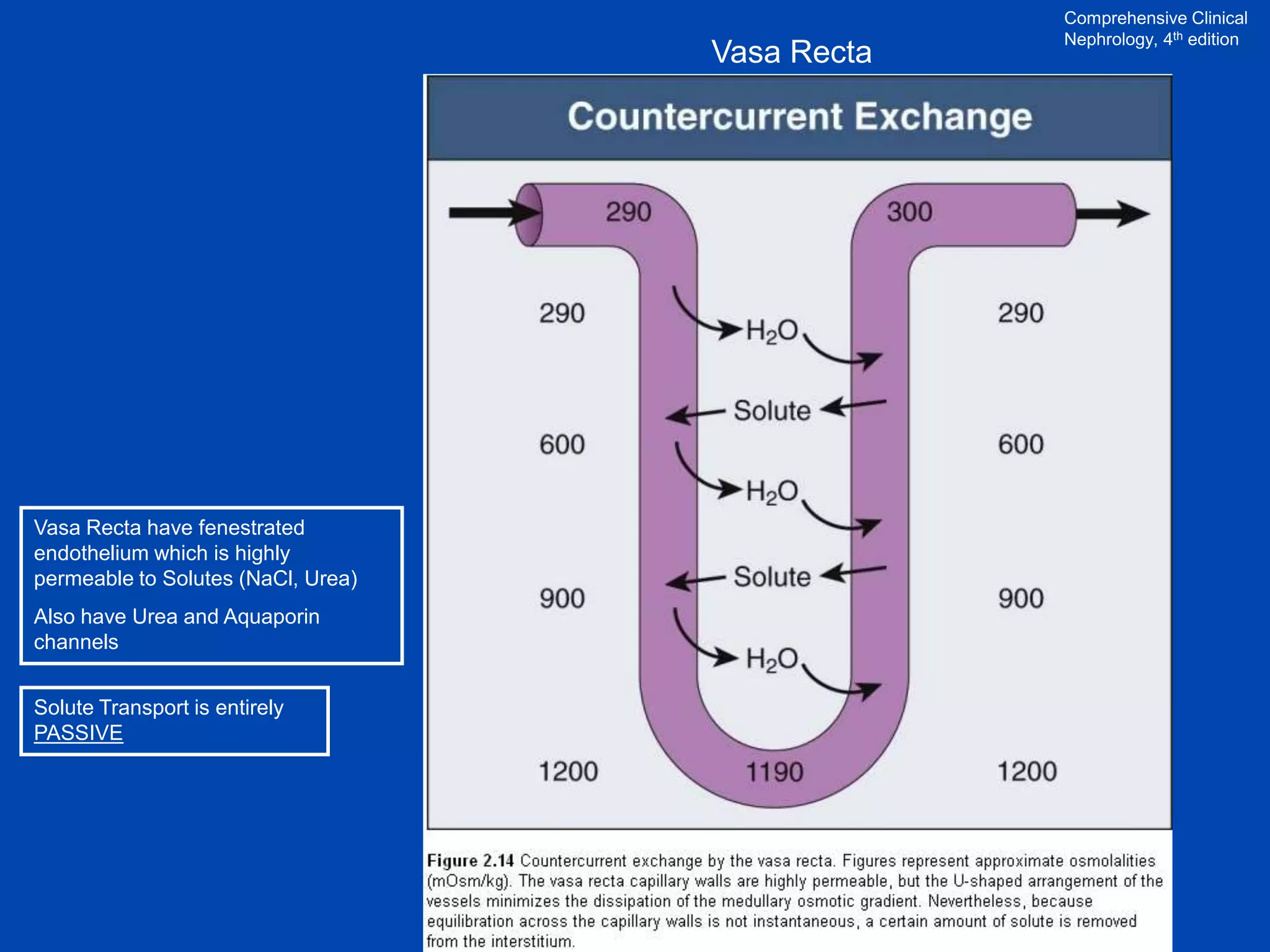
Vasa
recta
600 mOsM
600 mOsM
700 mOsM
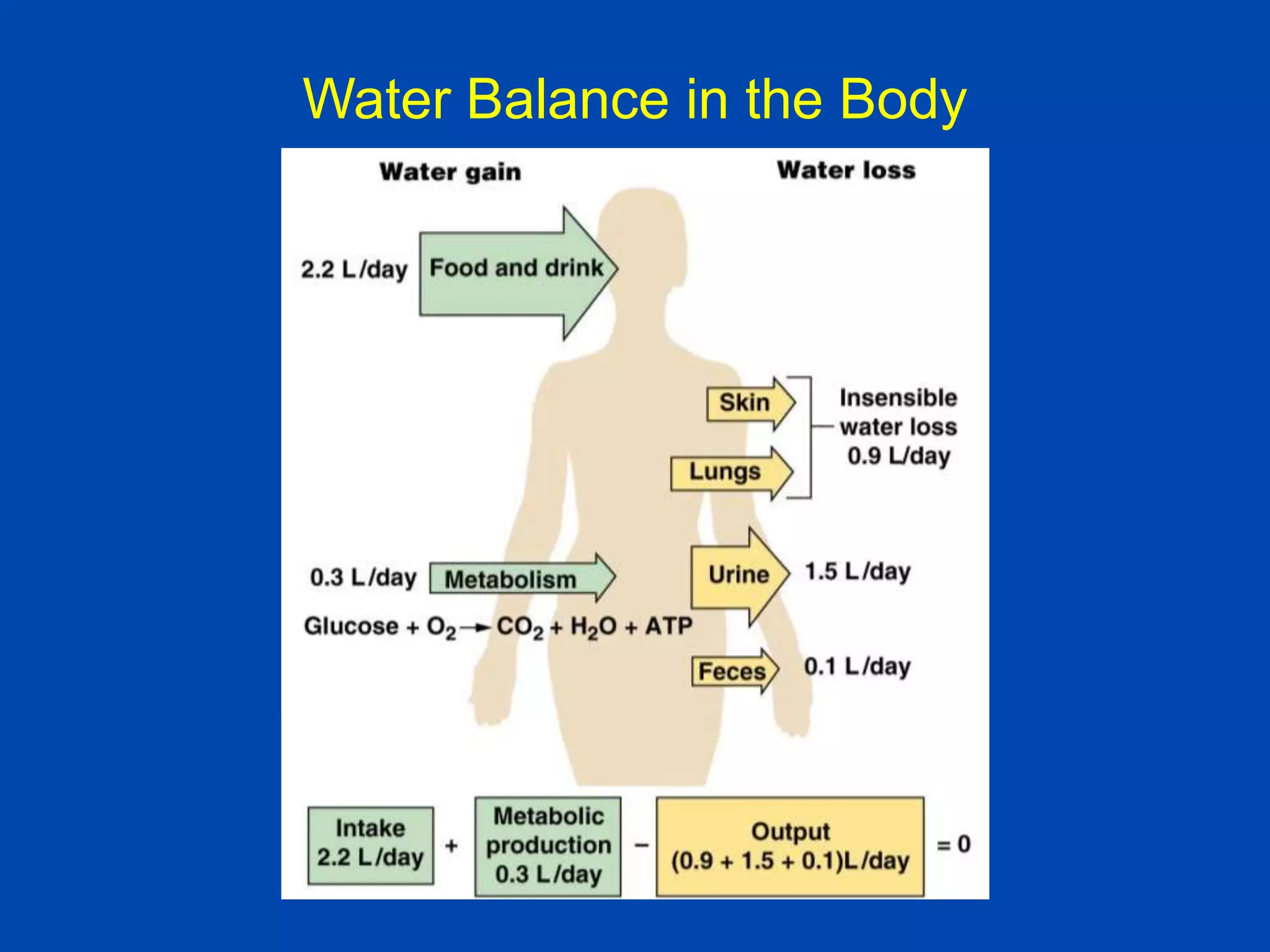
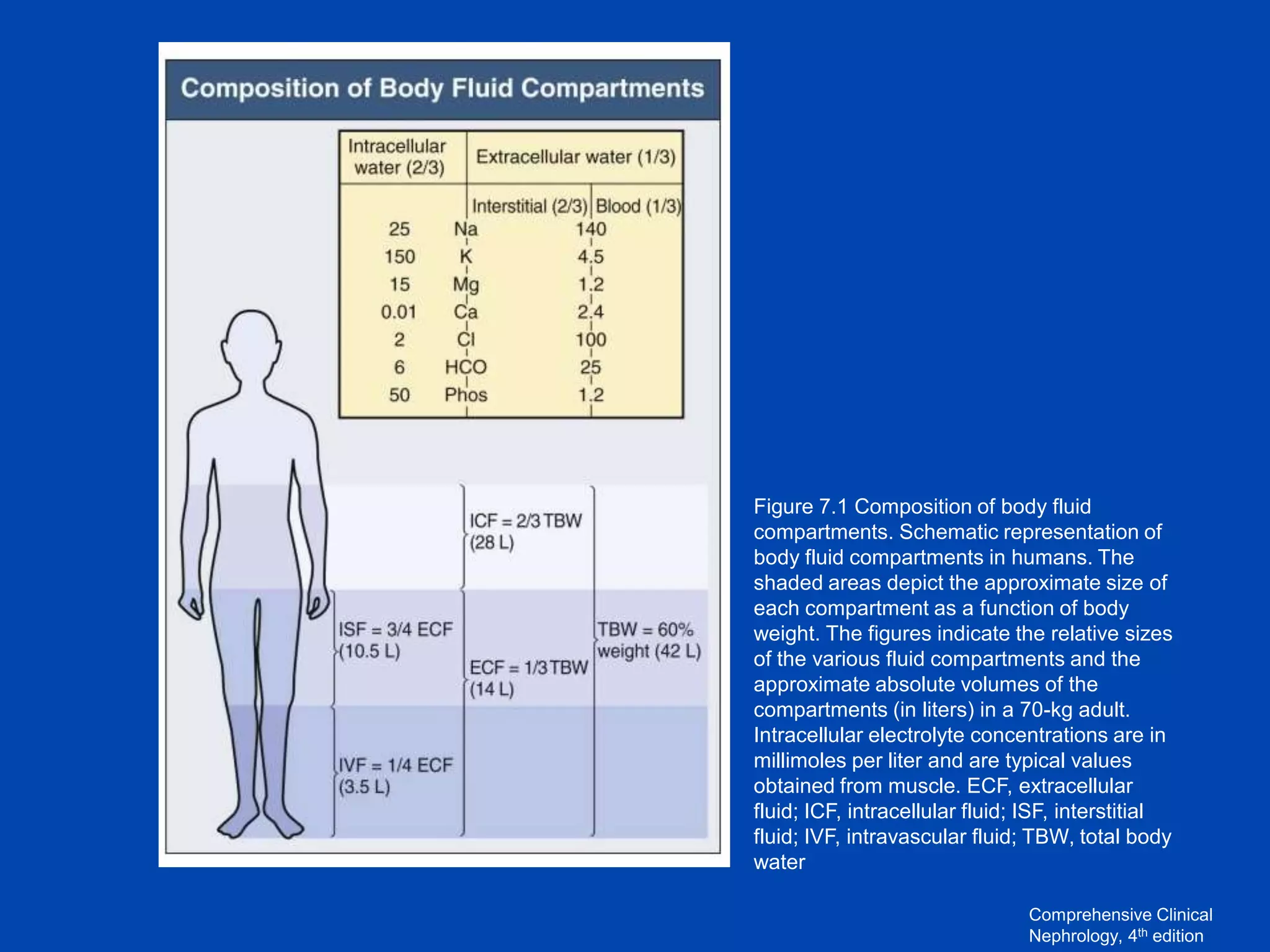
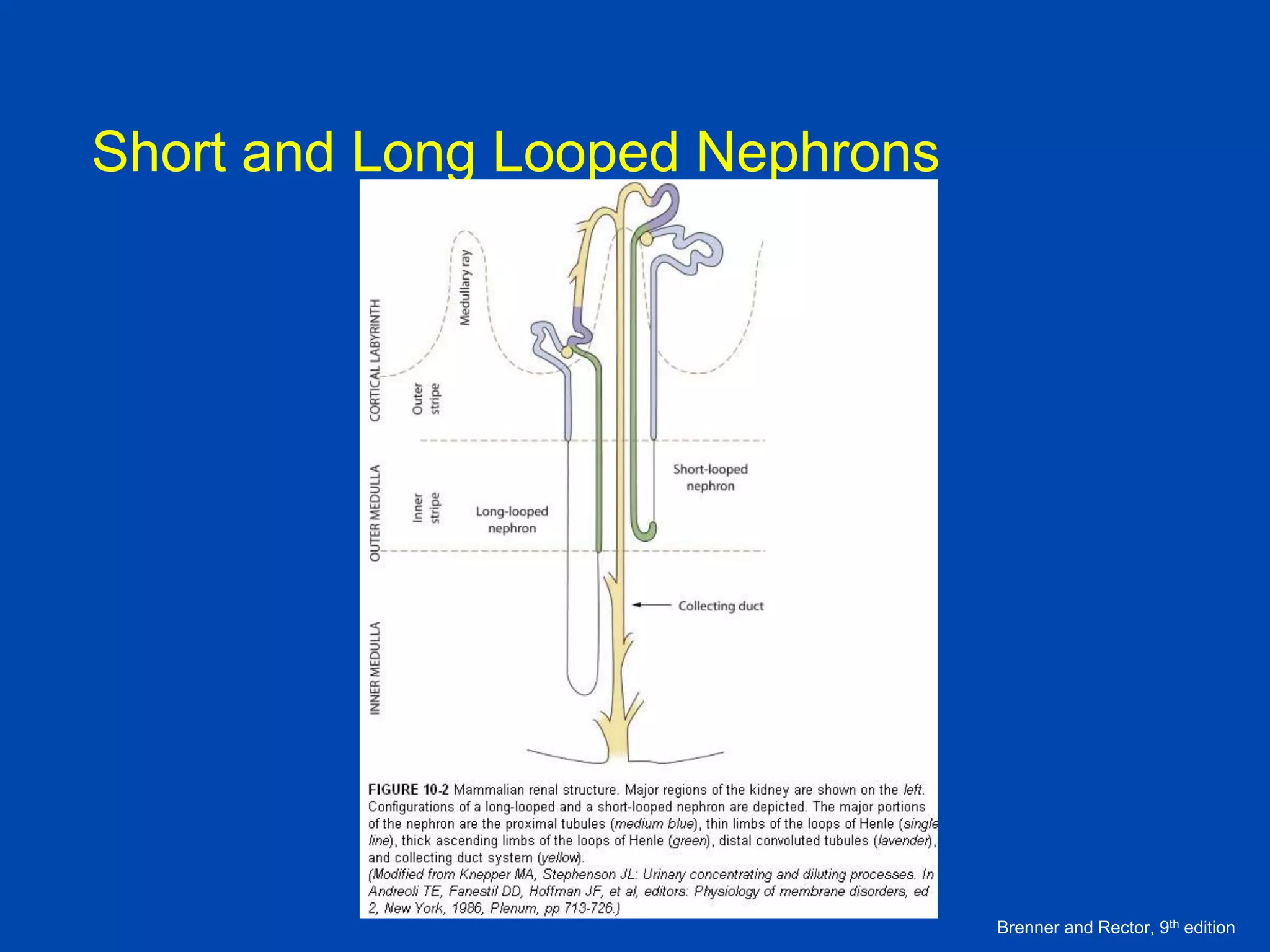
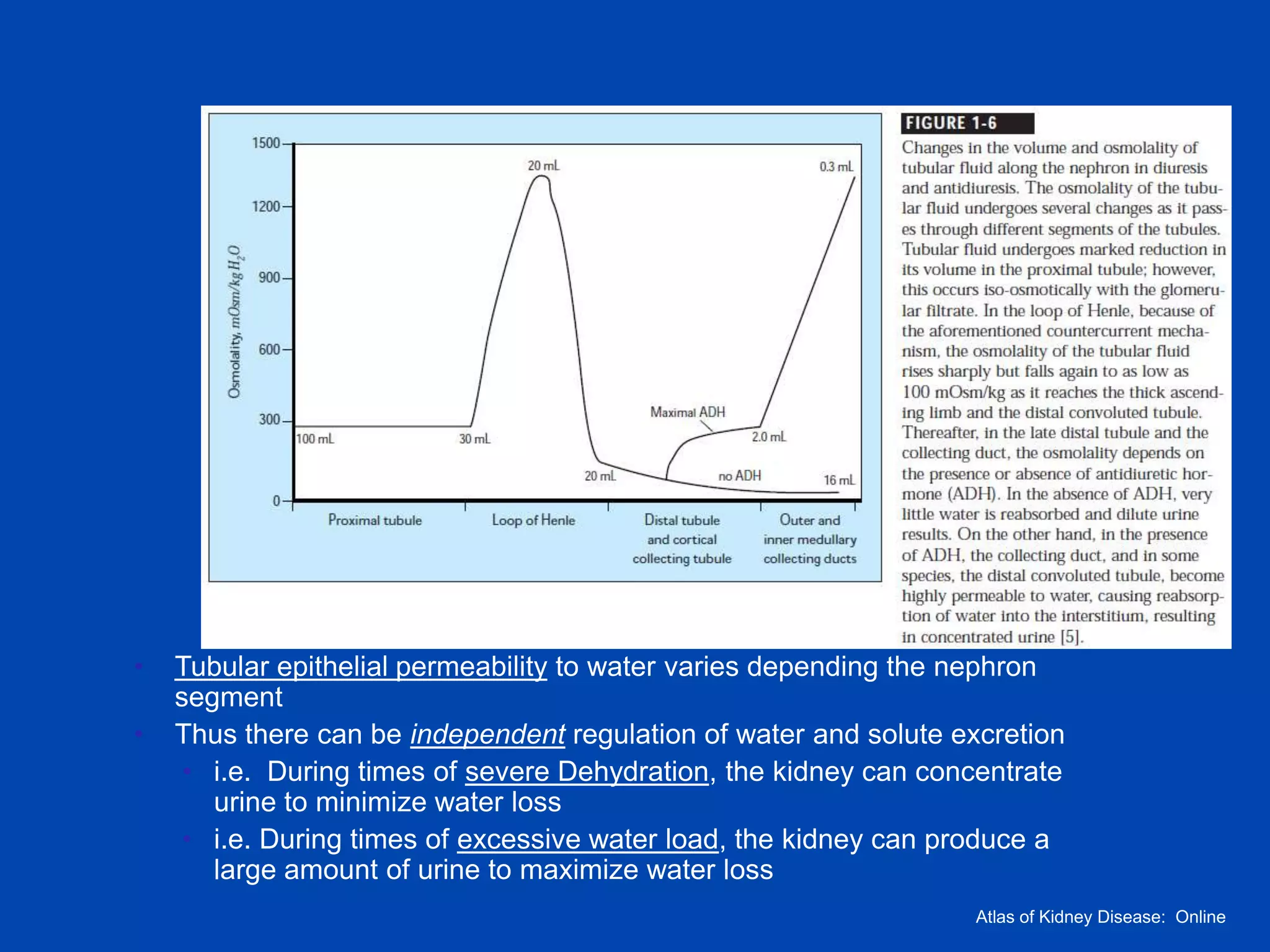
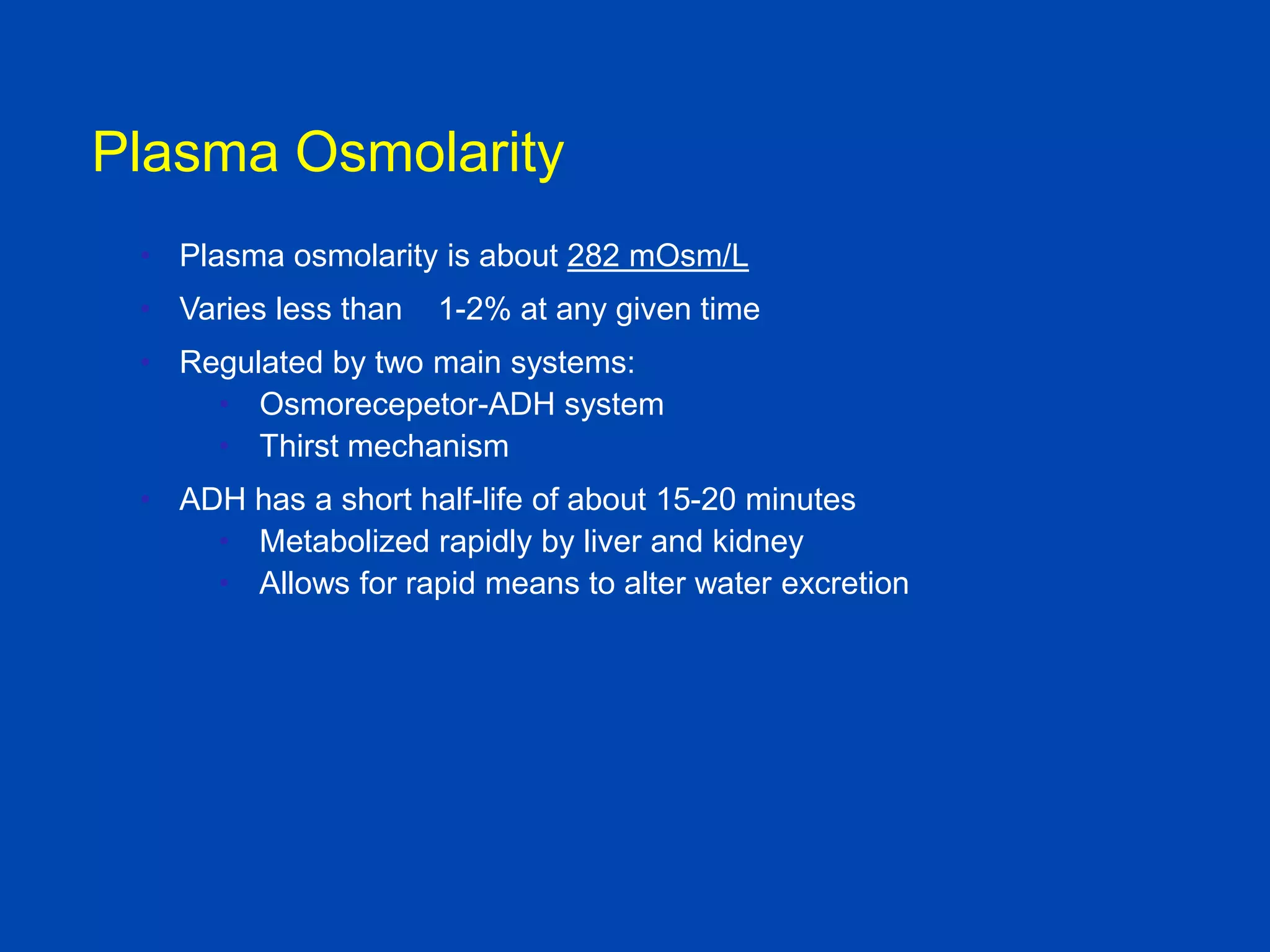
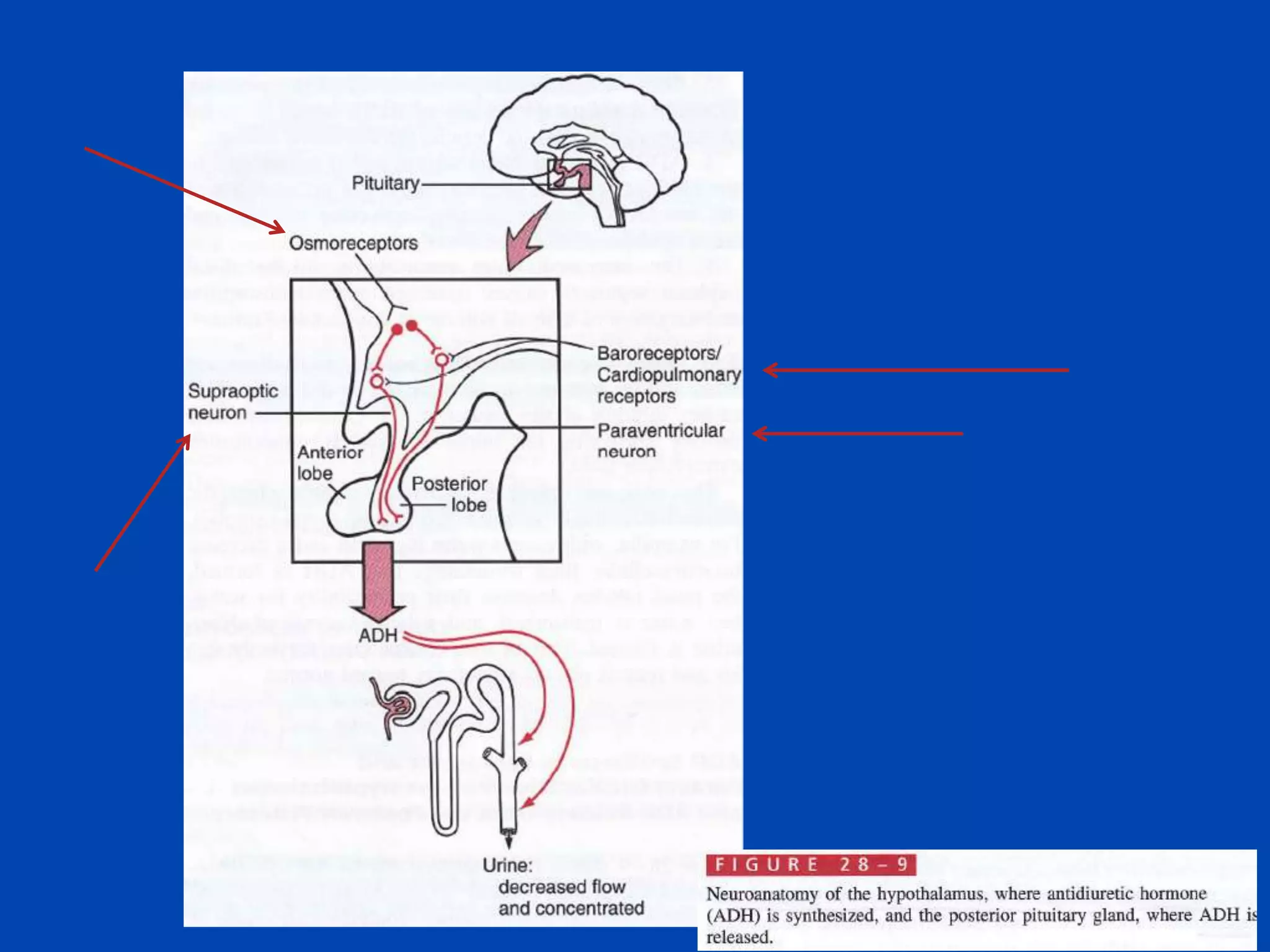
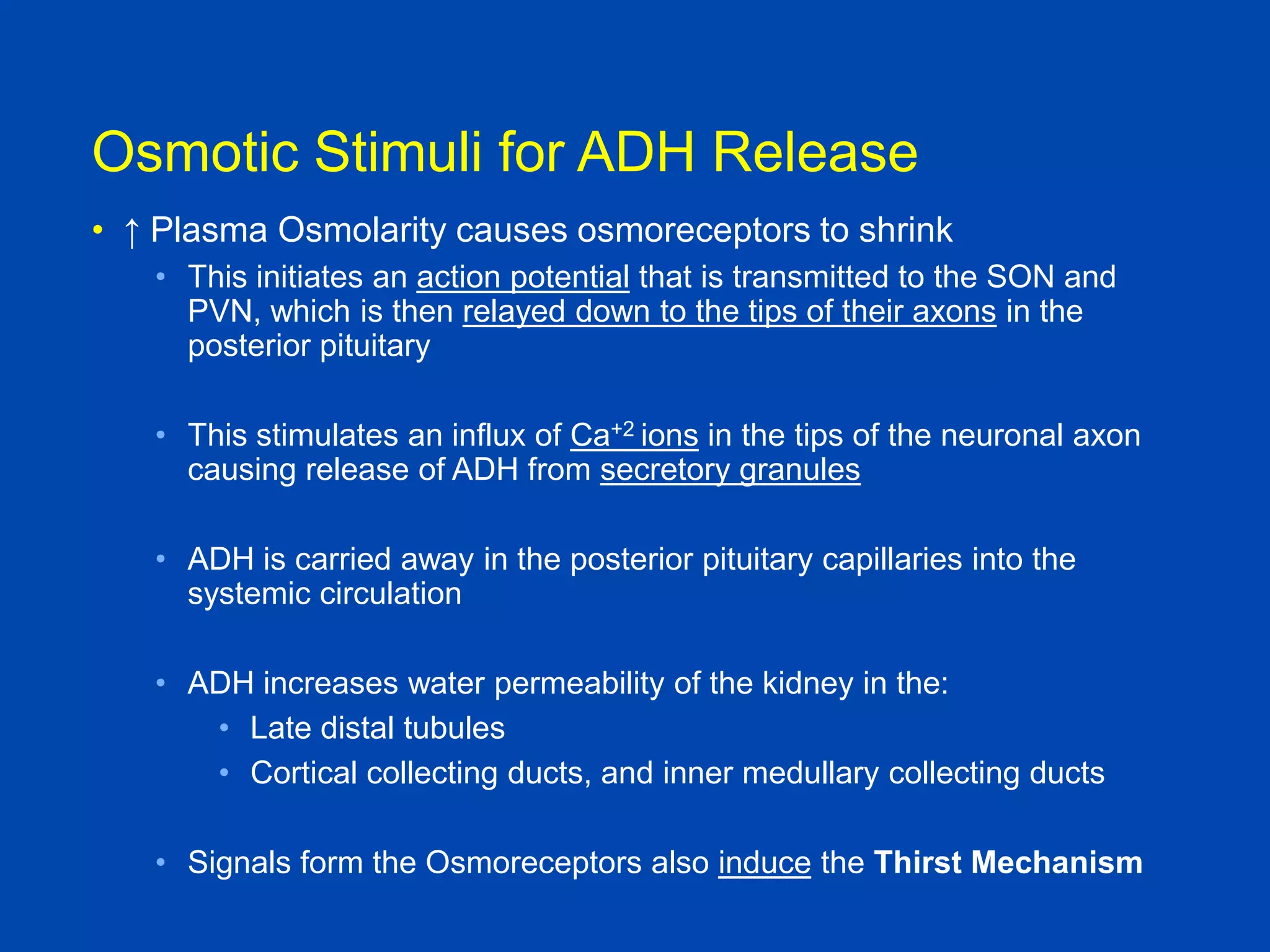
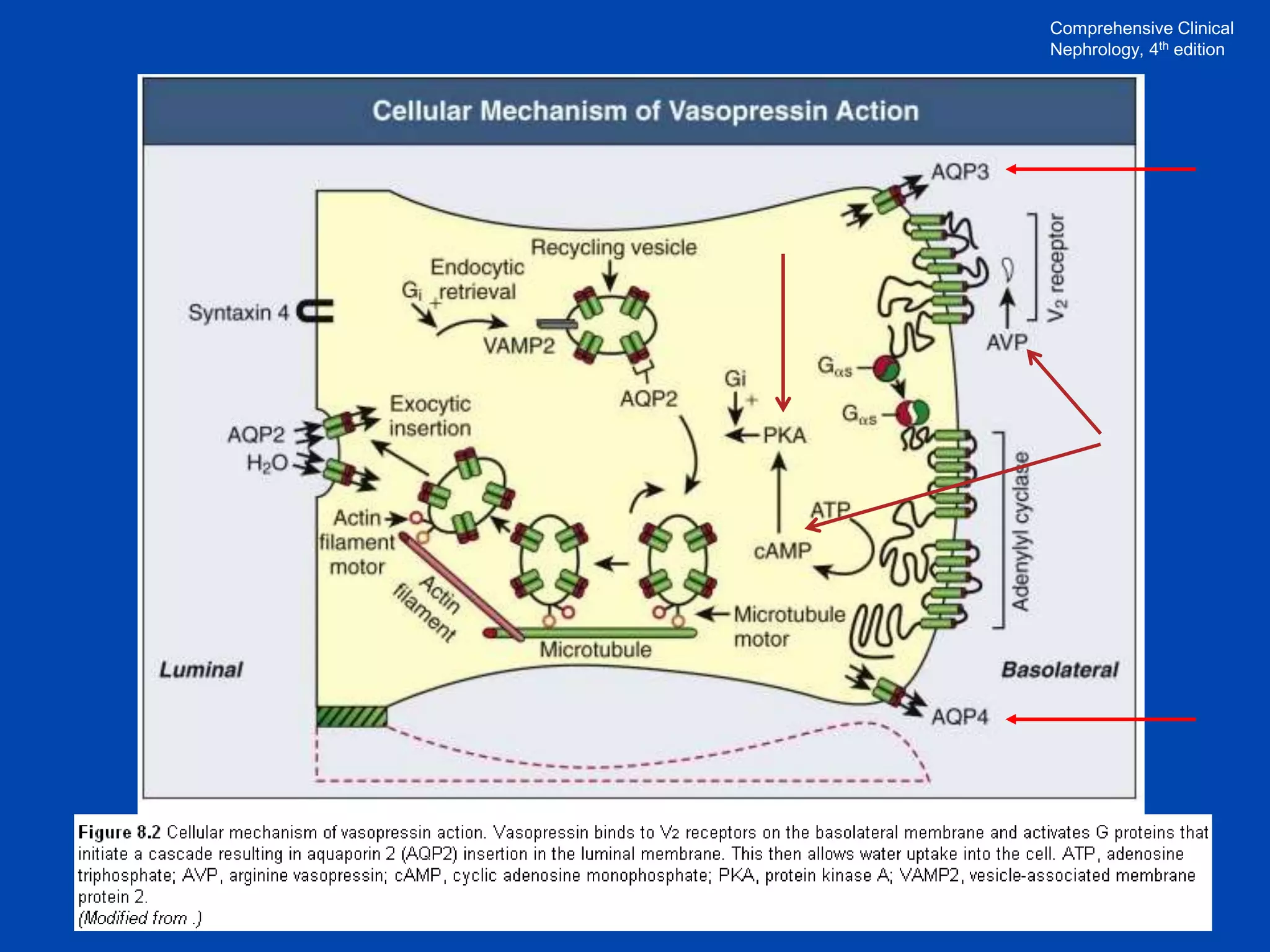
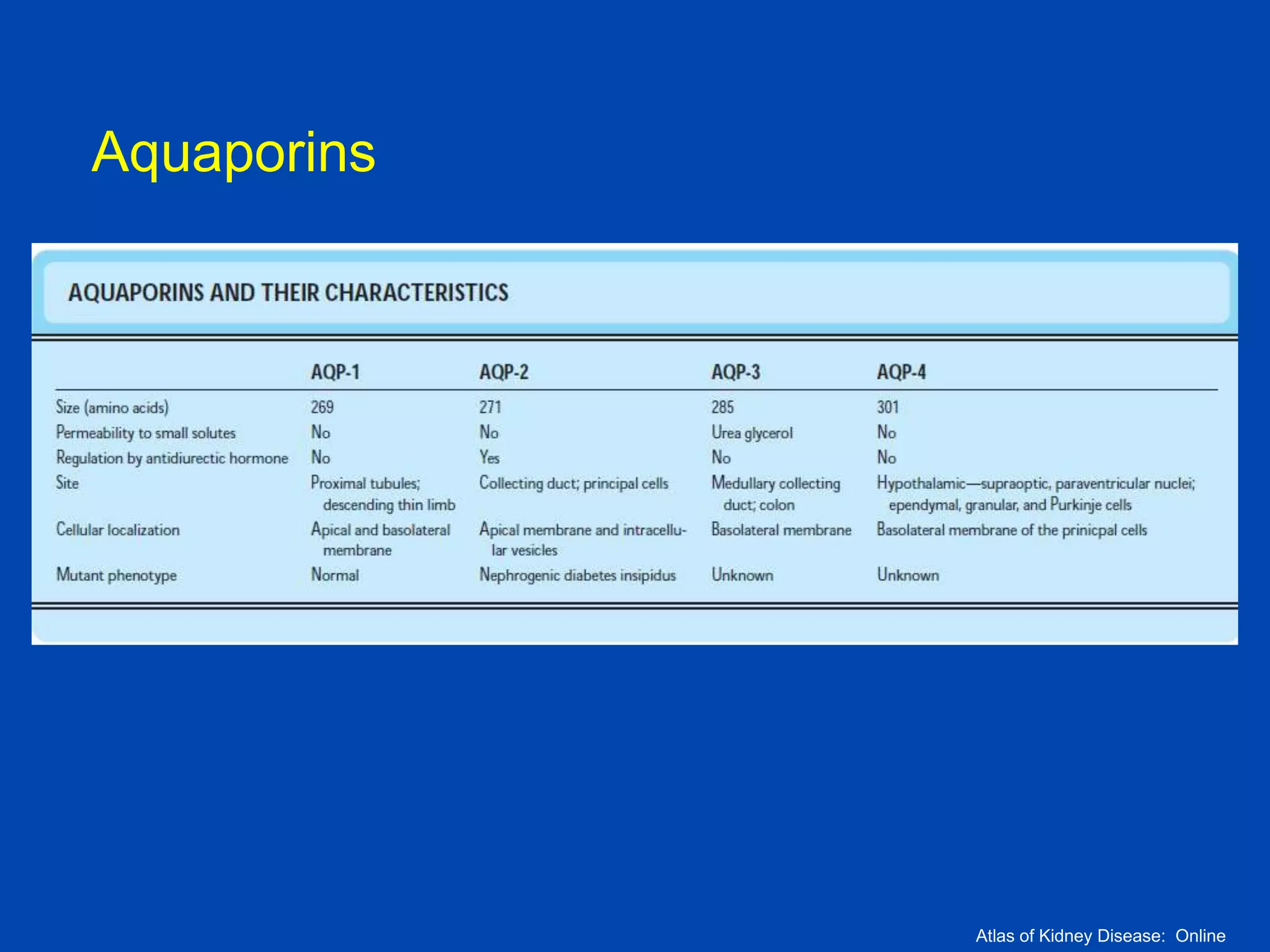
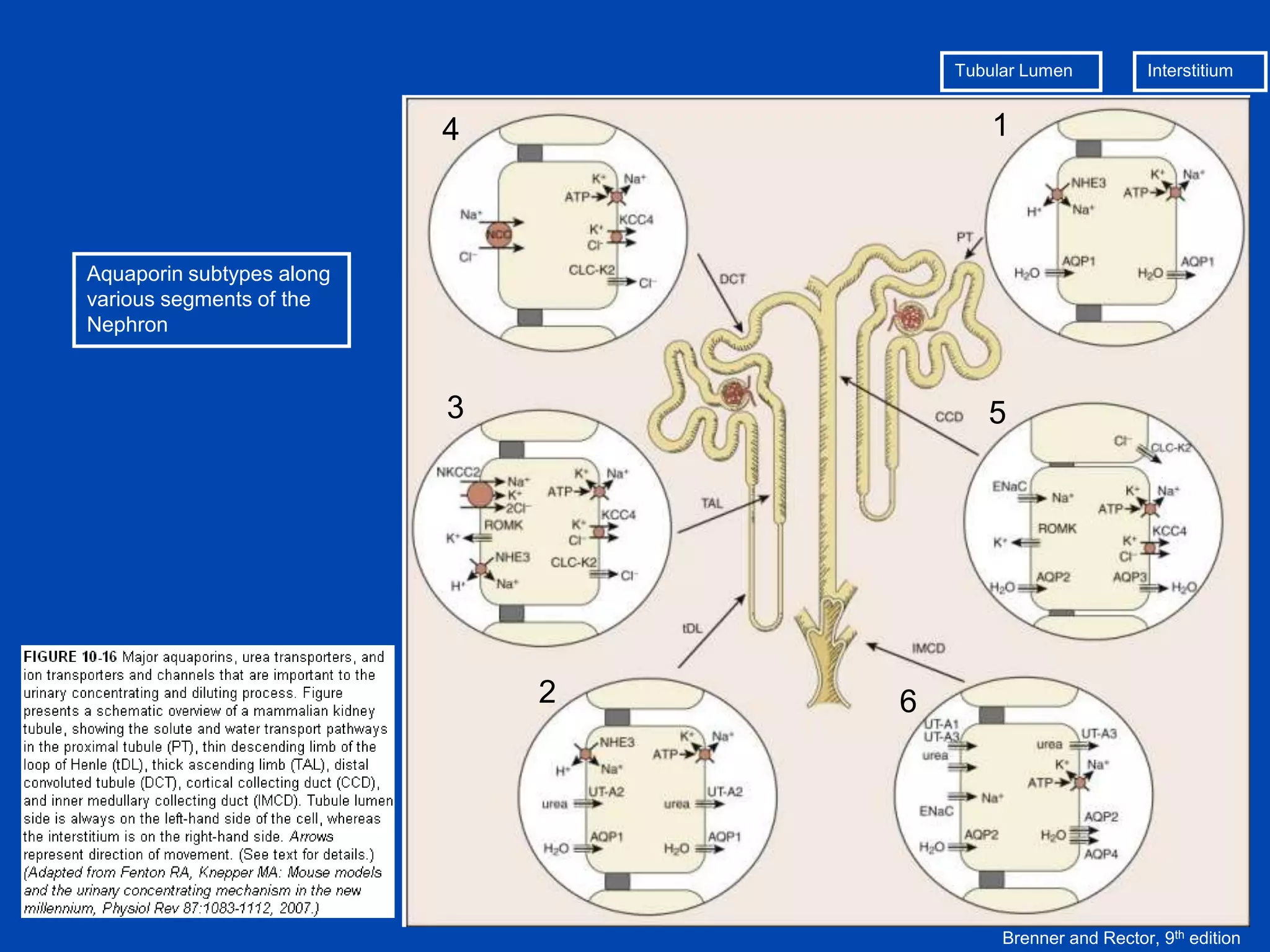
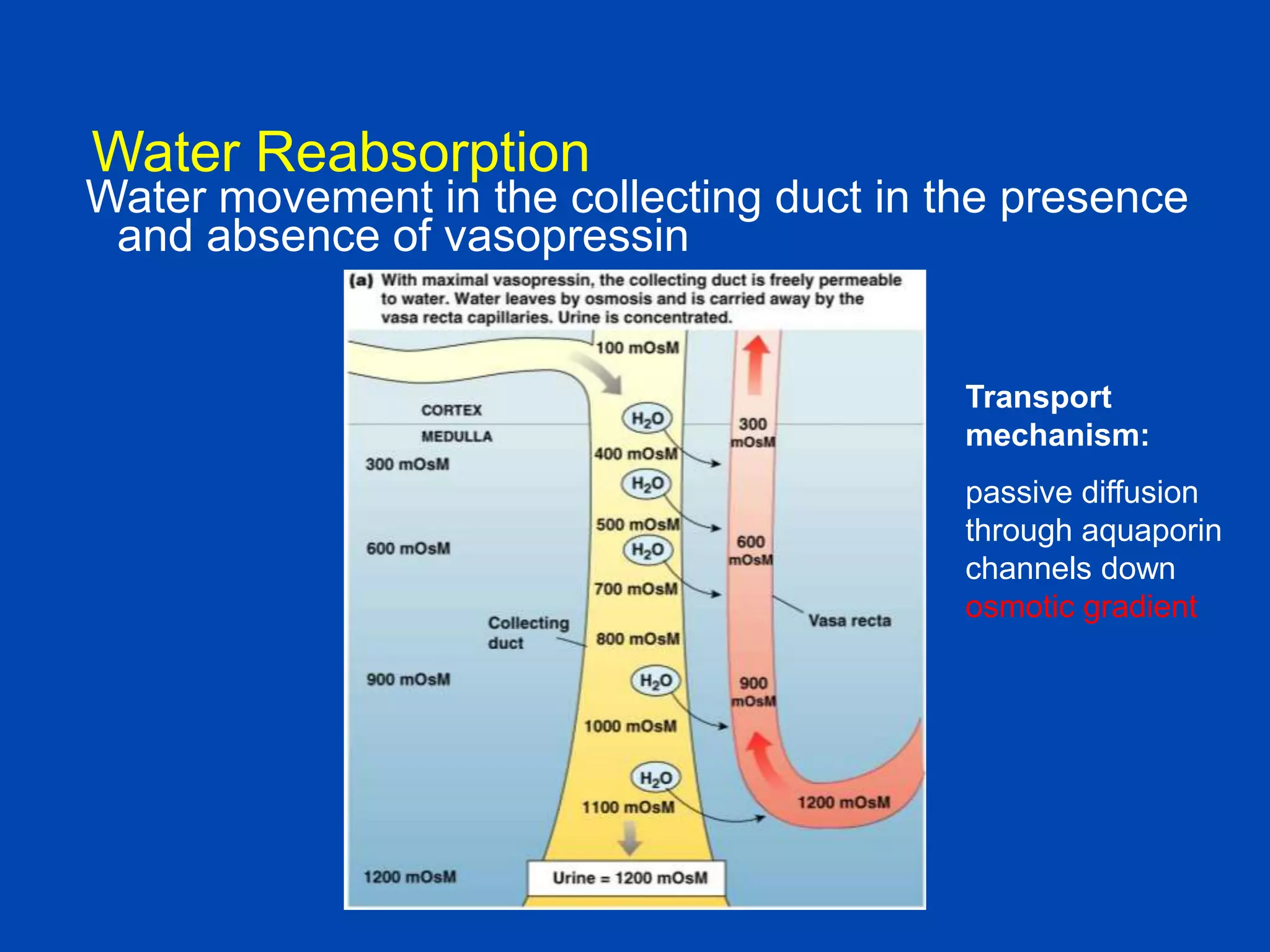
1. The kidney plays a pivotal role in maintaining water homeostasis by conserving water during deprivation and excreting excess water. This is accomplished through a complex anatomical arrangement of the nephron and renal vasculature.
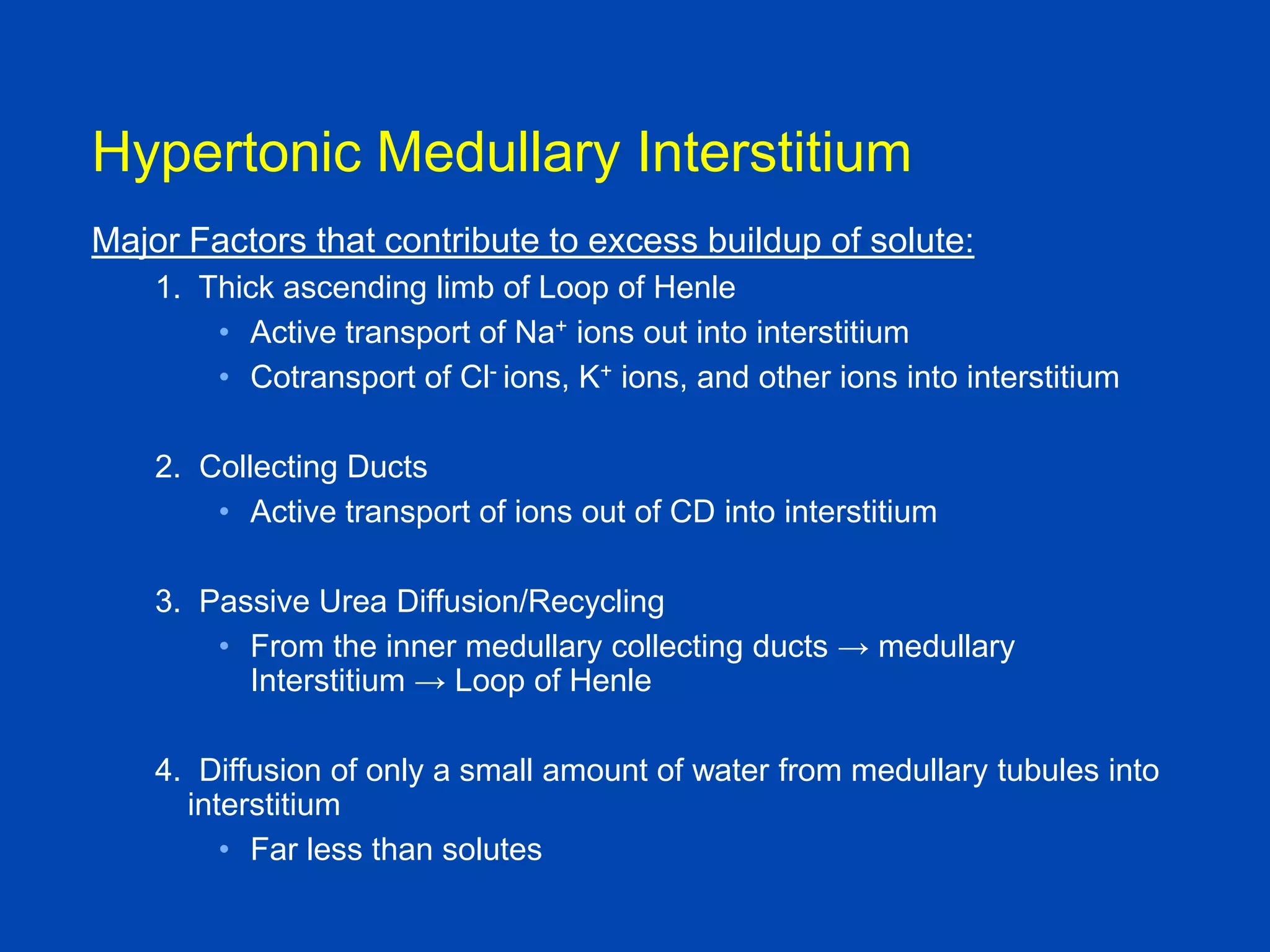
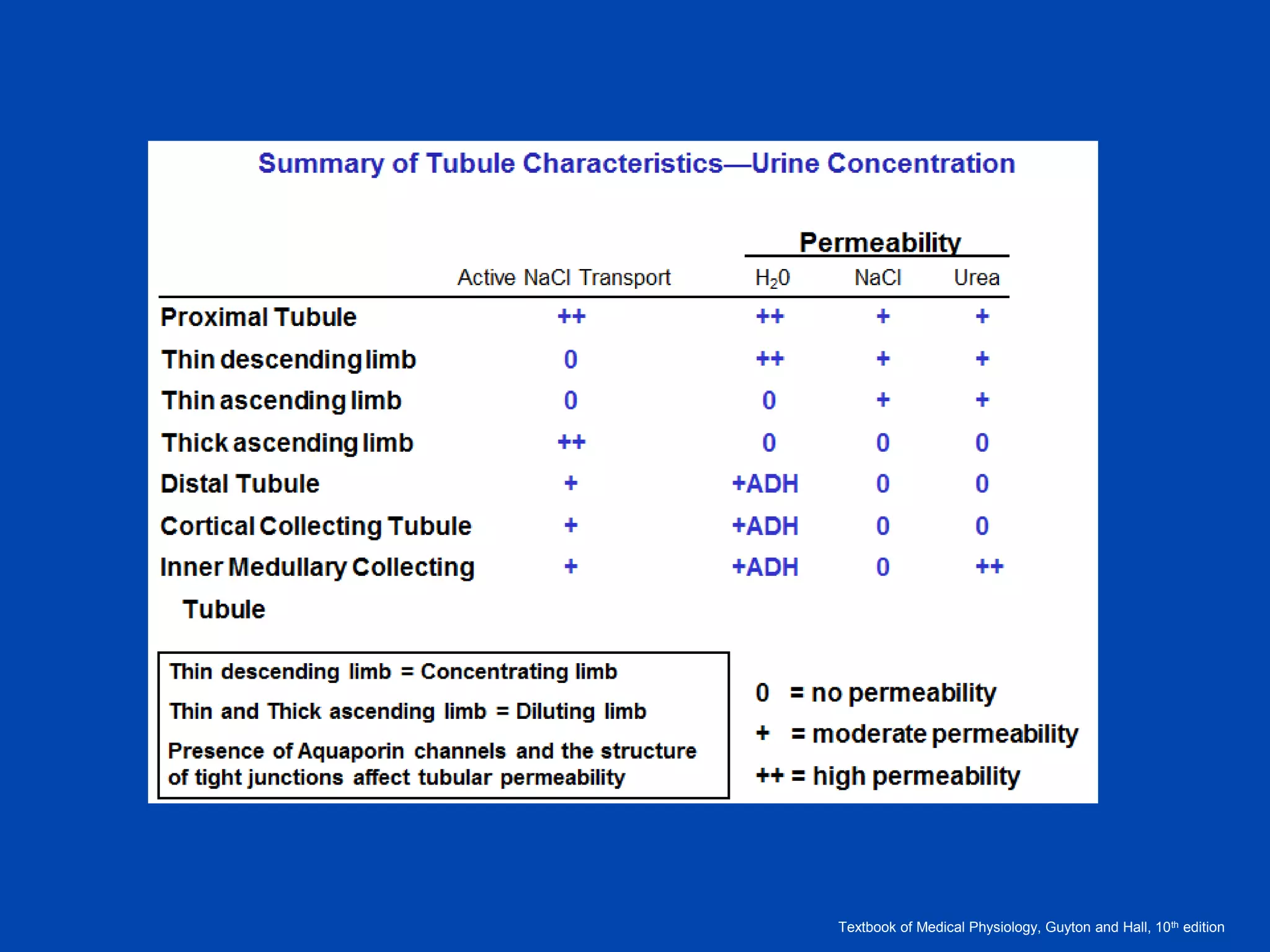
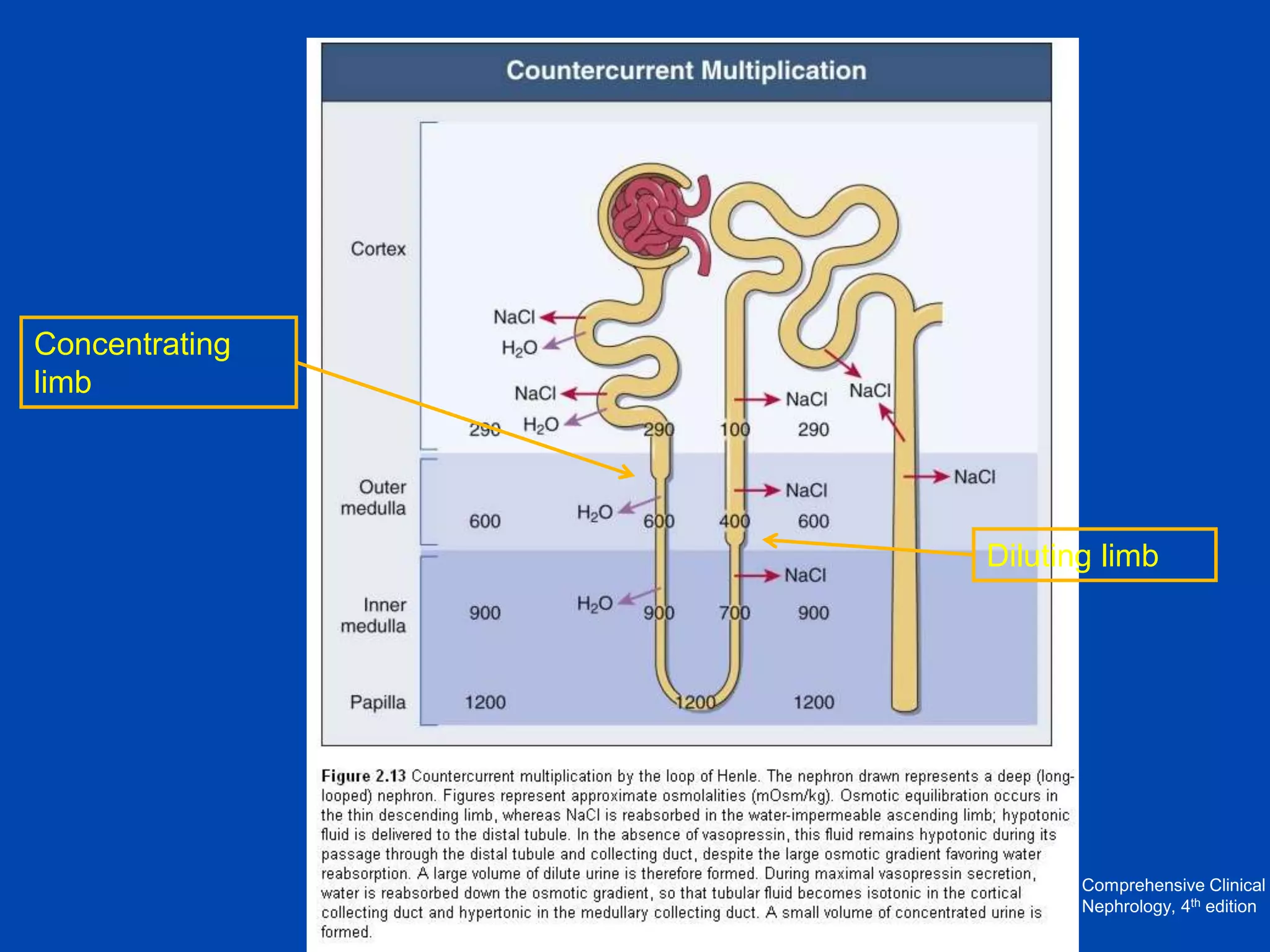
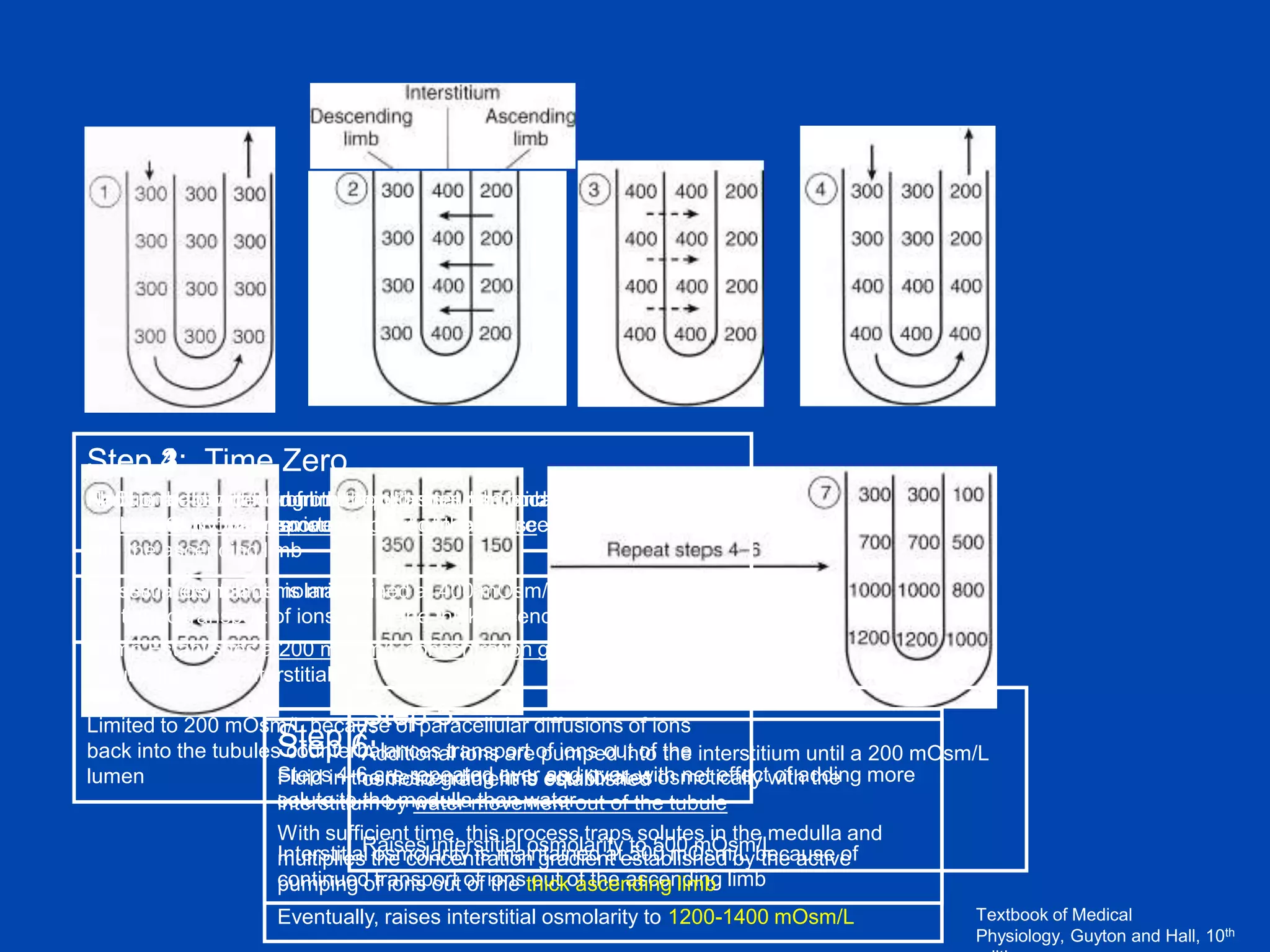
2. Key elements include a hypertonic medullary interstitium generated by active transport of solutes out of the thick ascending limb of the loop of Henle and collecting