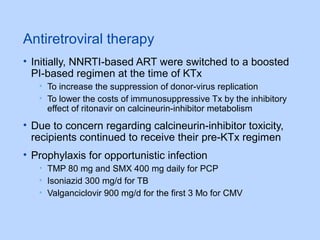
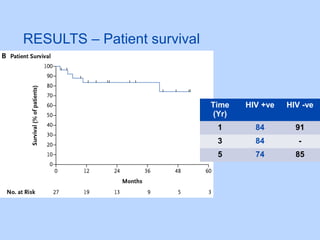
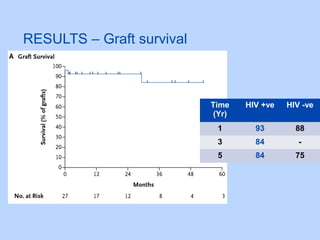
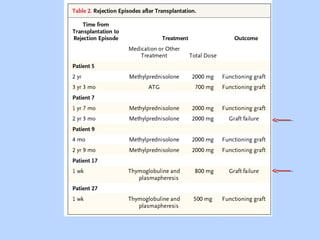
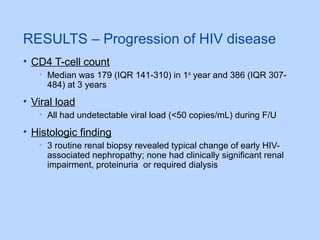
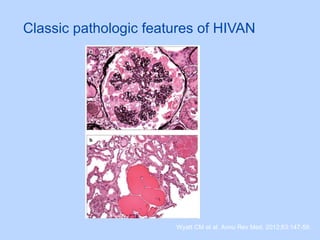
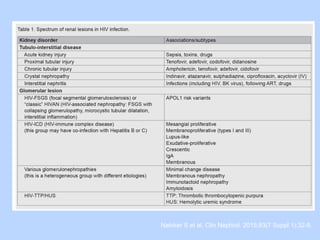
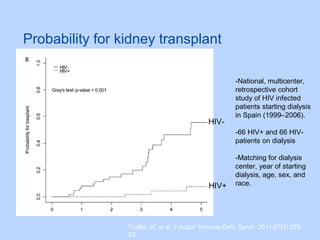
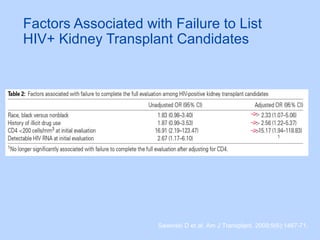
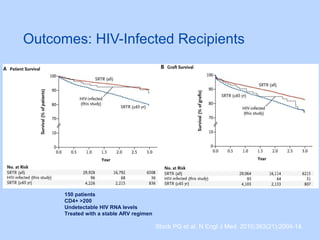
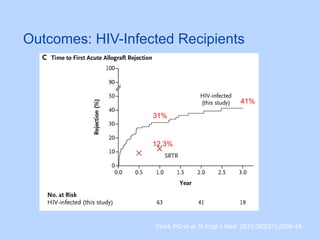
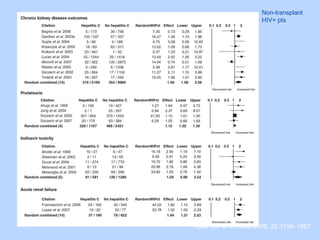
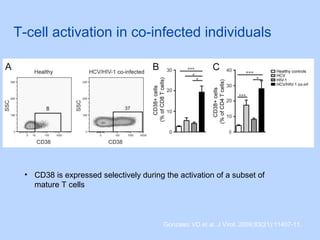
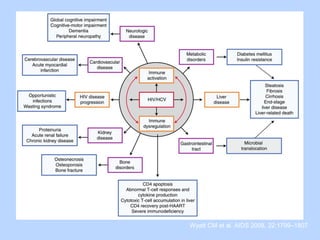
This document summarizes research on kidney transplantation between HIV-positive donors and recipients. A study in South Africa found that outcomes for 27 HIV-positive recipients of kidneys from 15 deceased HIV-positive donors were comparable to recipients of HIV-negative organs over 2-3 years. However, applying this approach in the U.S. may pose challenges due to higher rates of antiretroviral resistance and limited access to dialysis in South Africa altering risk-benefit calculations. While expanding access to transplant, ethics require caution given limited long-term outcomes data from HIV-positive donors.




![• SRTR; 2002–2011
• 510 adult kidney transplant recipients with HIV
(median follow-up, 3.8 years) matched 1:10 to
HIV-negative controls
Locke JE et al. J Am Soc Nephrol. 2015. [Epub ahead of print]](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-15-320.jpg)
![The number of kidney transplants
Locke JE et al. J Am Soc Nephrol. 2015. [Epub ahead of print]](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-16-320.jpg)
![Graft survival
Locke JE et al. J Am Soc Nephrol. 2015. [Epub ahead of print]
HR 1.06; 95% CI, 0.85-1.33; P=0.61
HIV-/HCV-
HIV+/HCV-](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-17-320.jpg)
![Graft survival
Locke JE et al. J Am Soc Nephrol. 2015. [Epub ahead of print]
HR 1.38; 95% CI, 1.08-1.77; P=0.01
HIV-/HCV+
HIV+/HCV+](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-18-320.jpg)
![Patient survival
Locke JE et al. J Am Soc Nephrol. 2015. [Epub ahead of print]
HIV-/HCV-
HIV+/HCV-](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-19-320.jpg)
![Patient survival
Locke JE et al. J Am Soc Nephrol. 2015. [Epub ahead of print]
HIV-/HCV+
HIV+/HCV+](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-20-320.jpg)


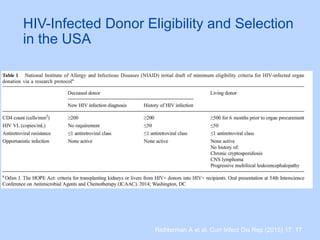

![• Nationally, approximate 356 potential HIV+
deceased donors yielding 192 kidneys and 247
livers annually.
Richterman A et al. Am J Transplant. 2015 May 14. [Epub ahead of print]](https://image.slidesharecdn.com/hivebm-150523175641-lva1-app6892/85/HIV-in-Kidney-Transplantation-28-320.jpg)