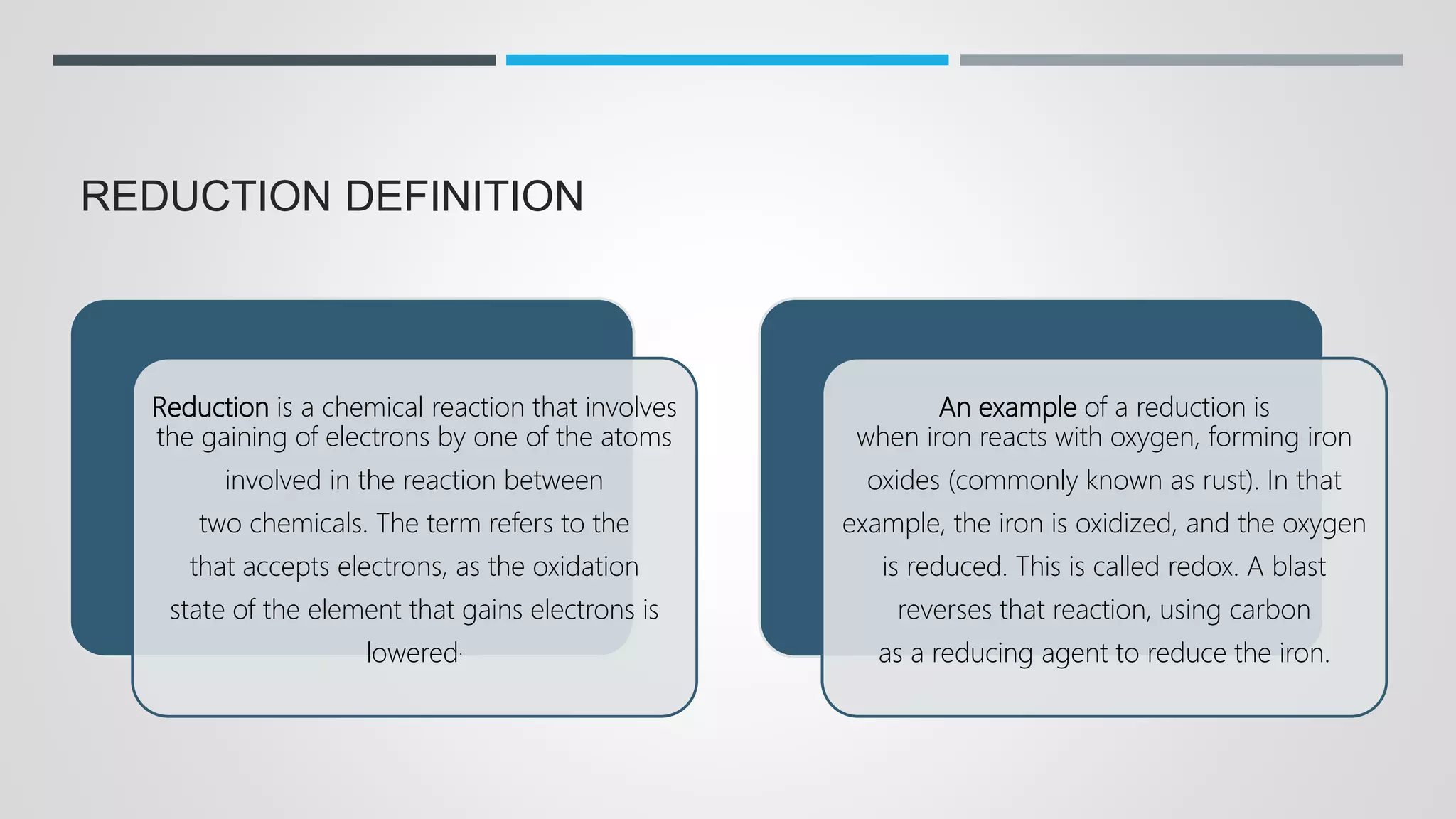
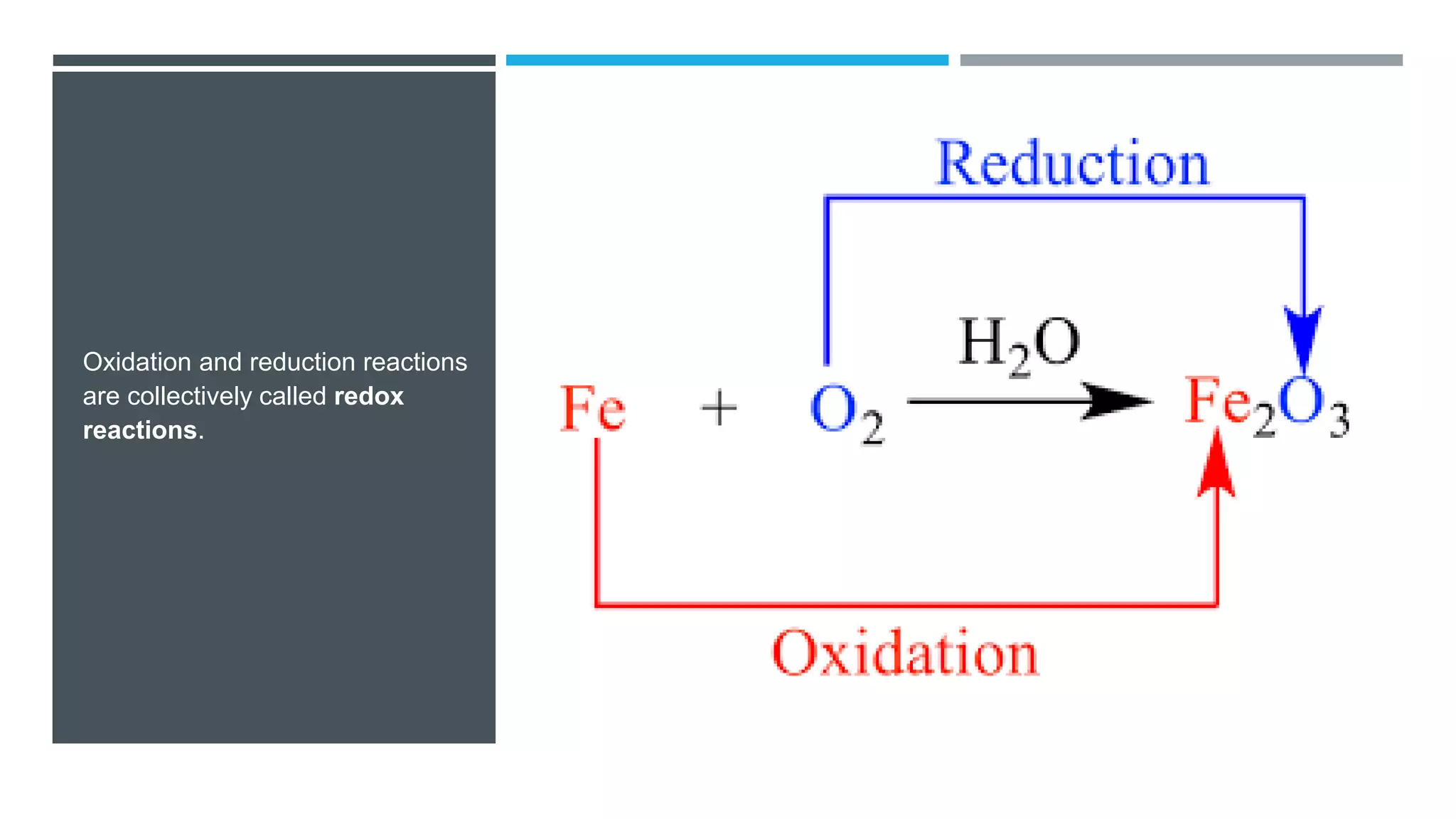
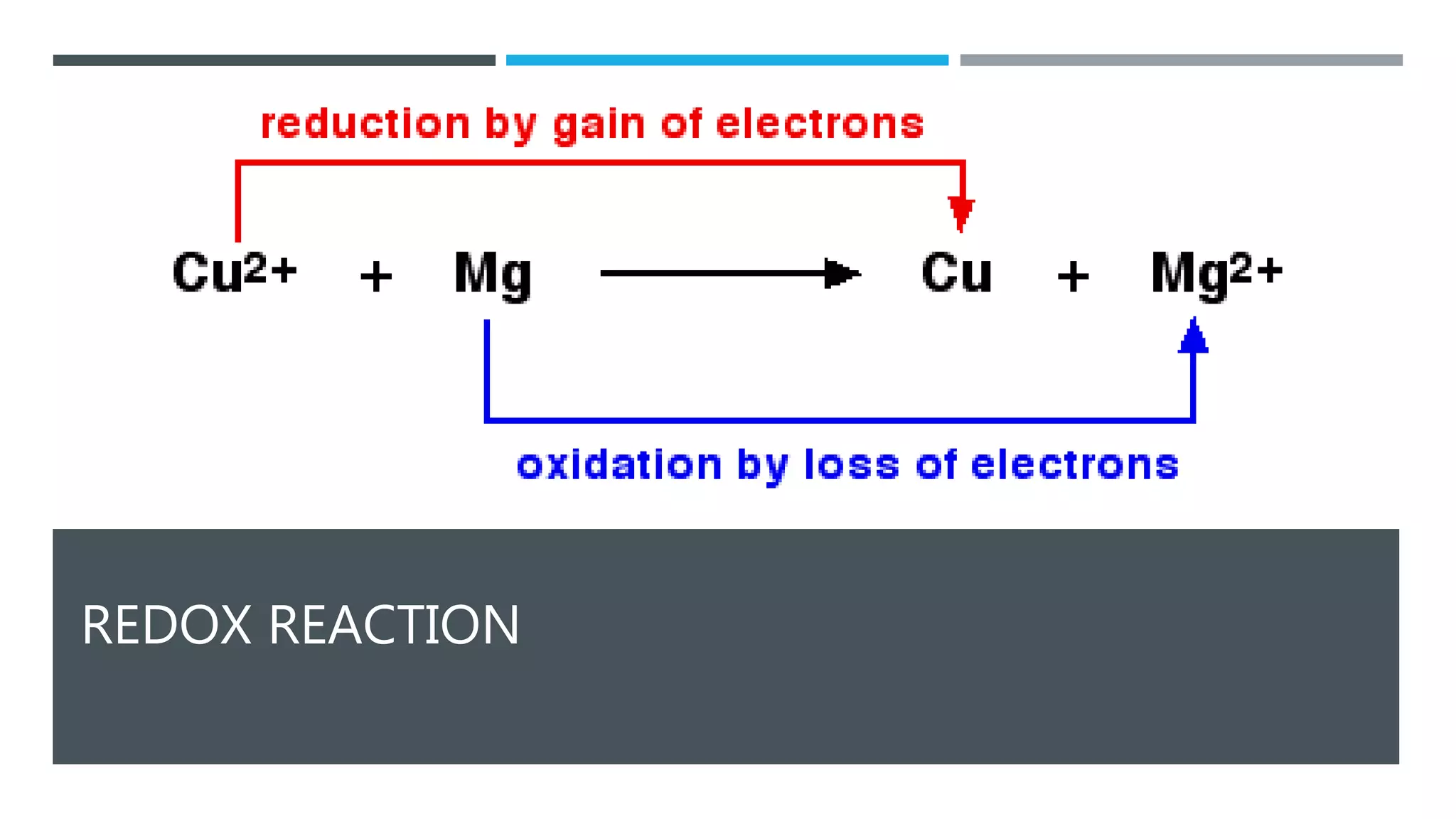
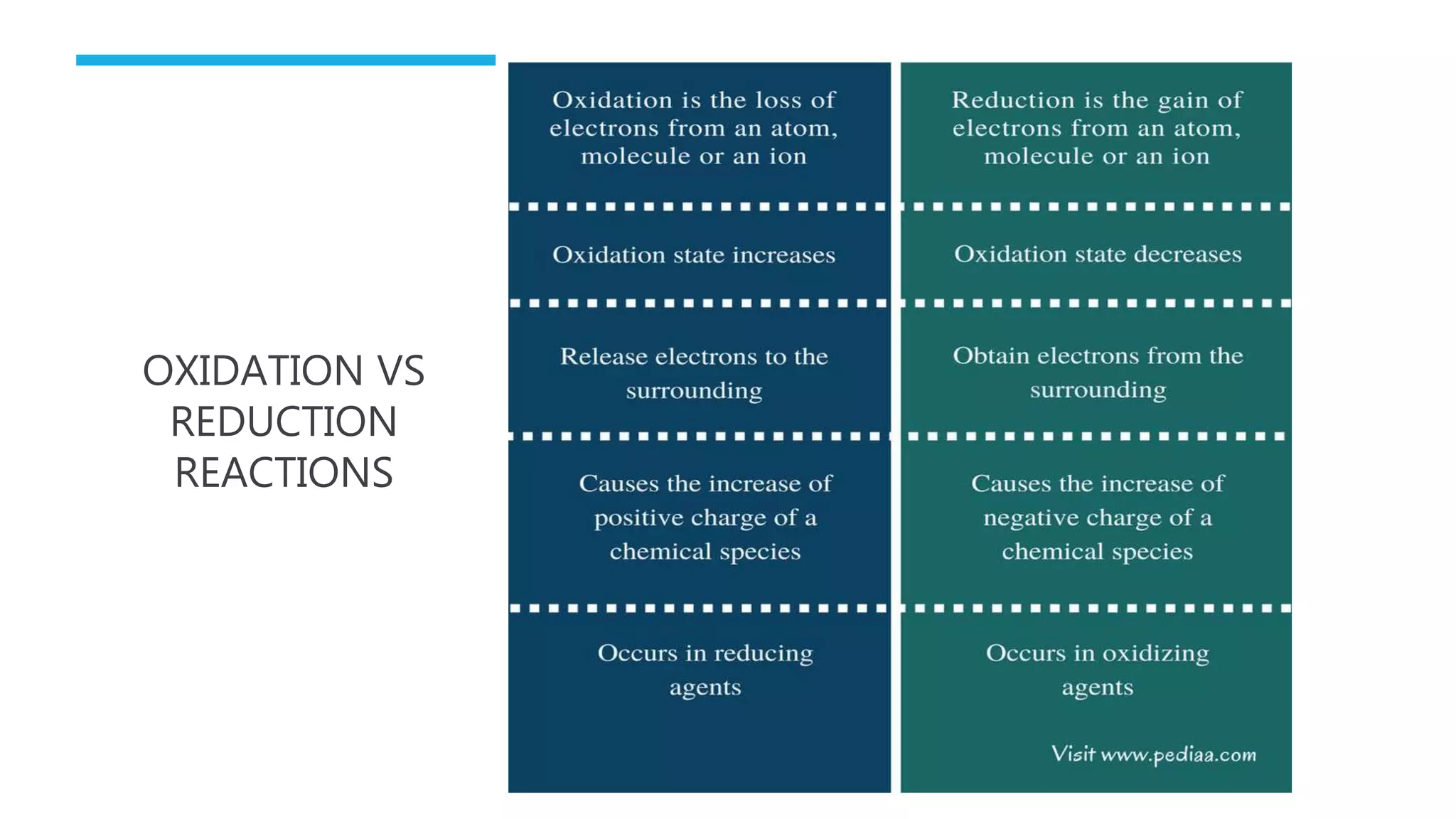
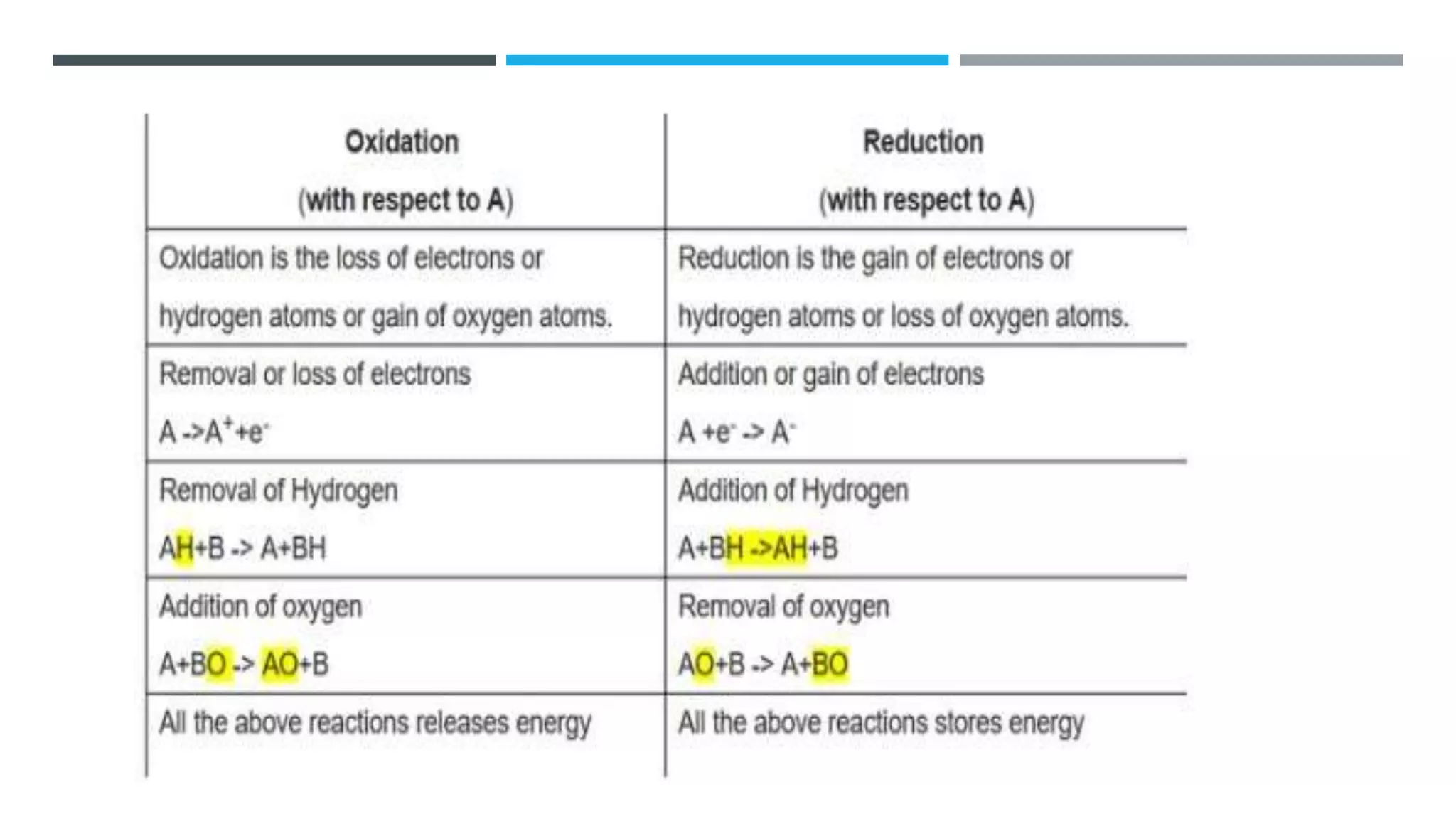
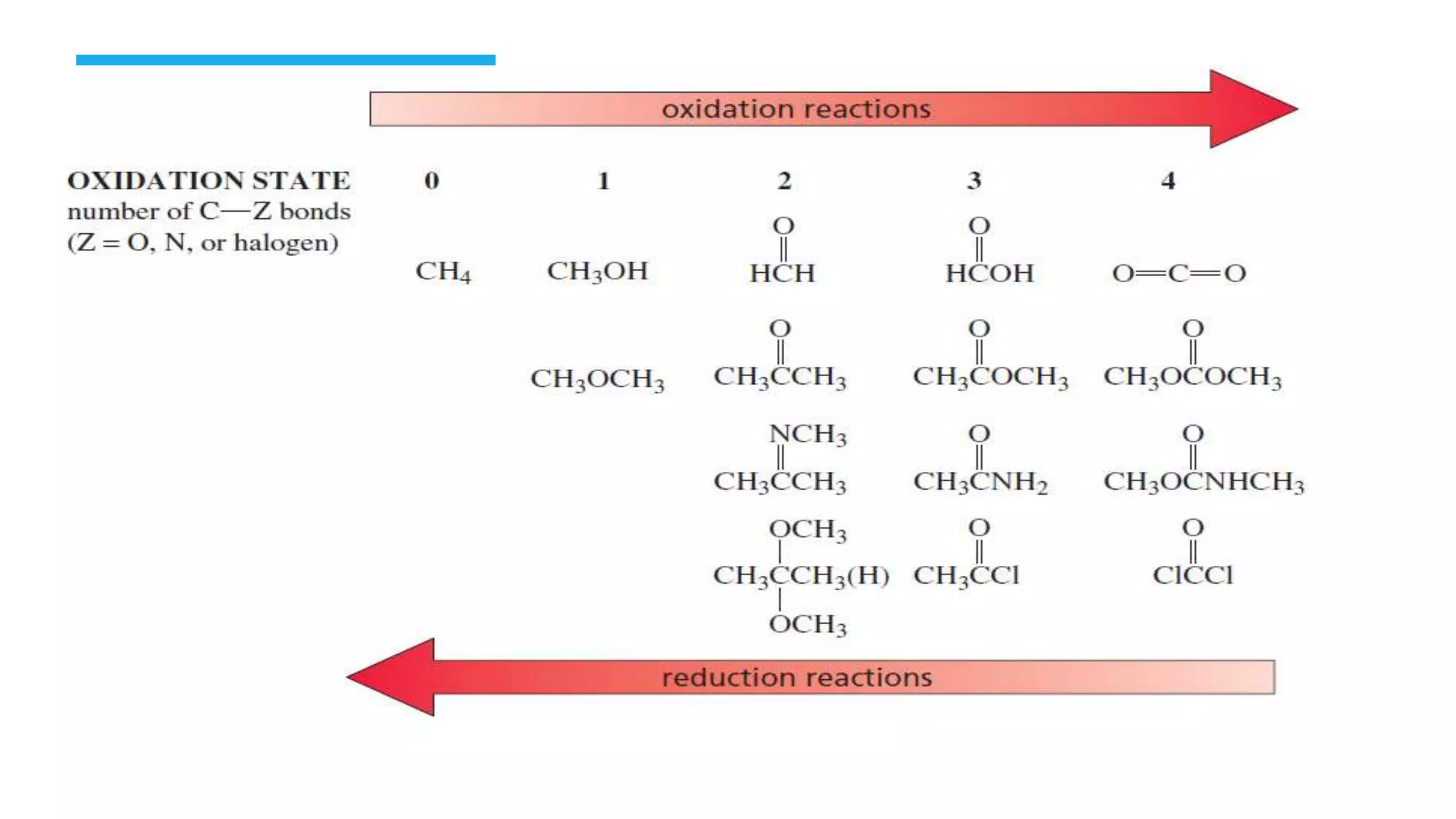
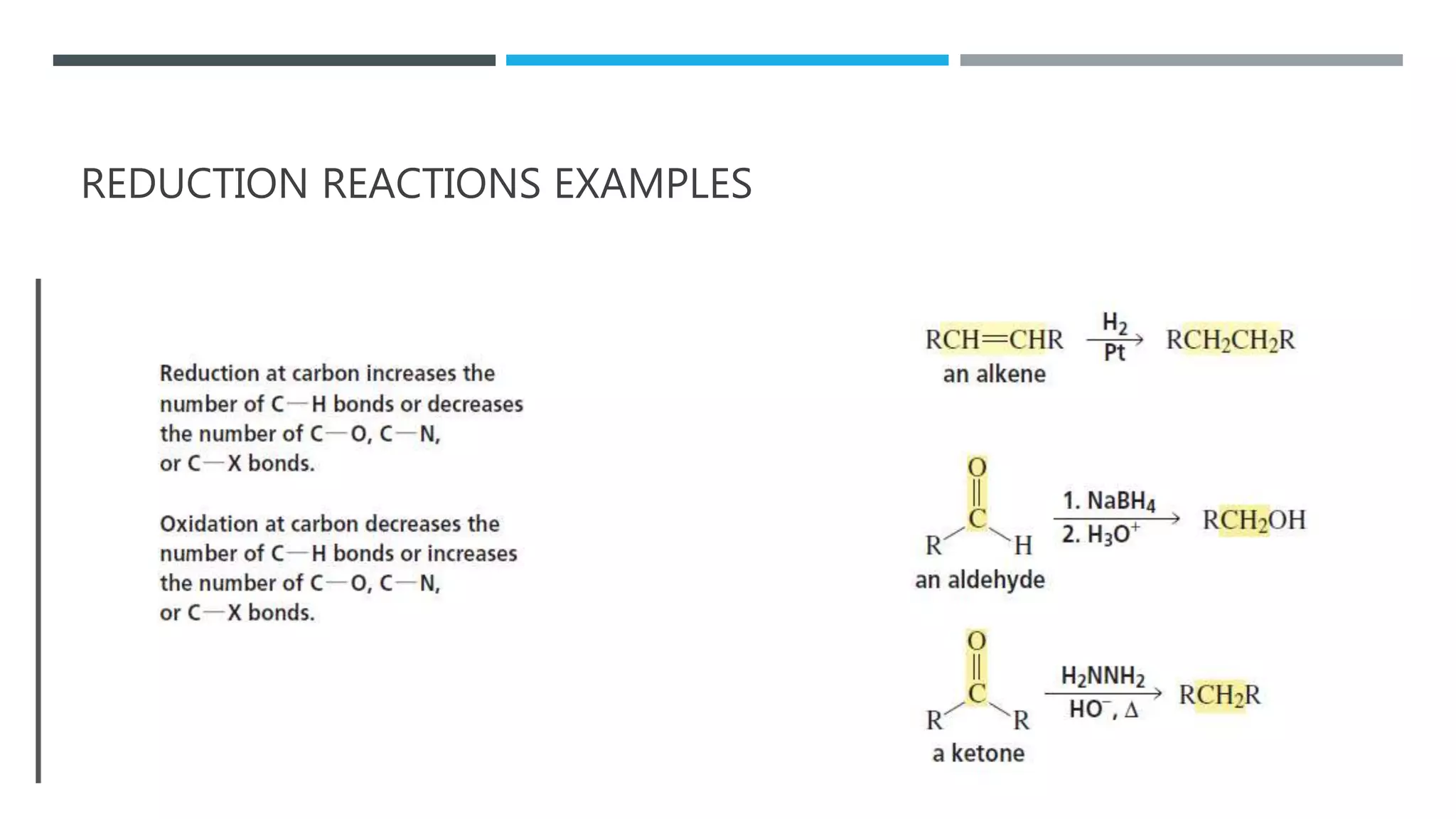
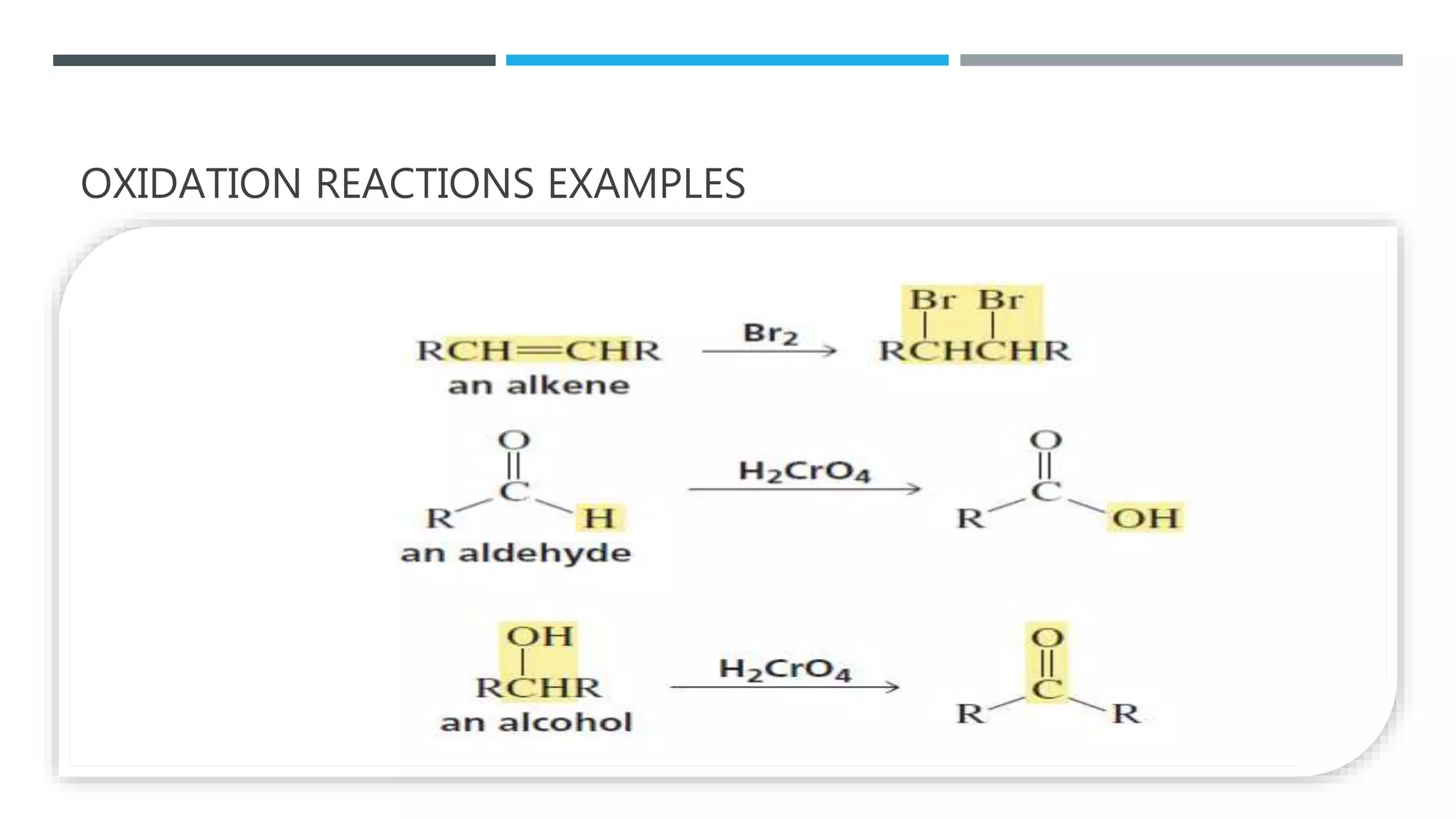
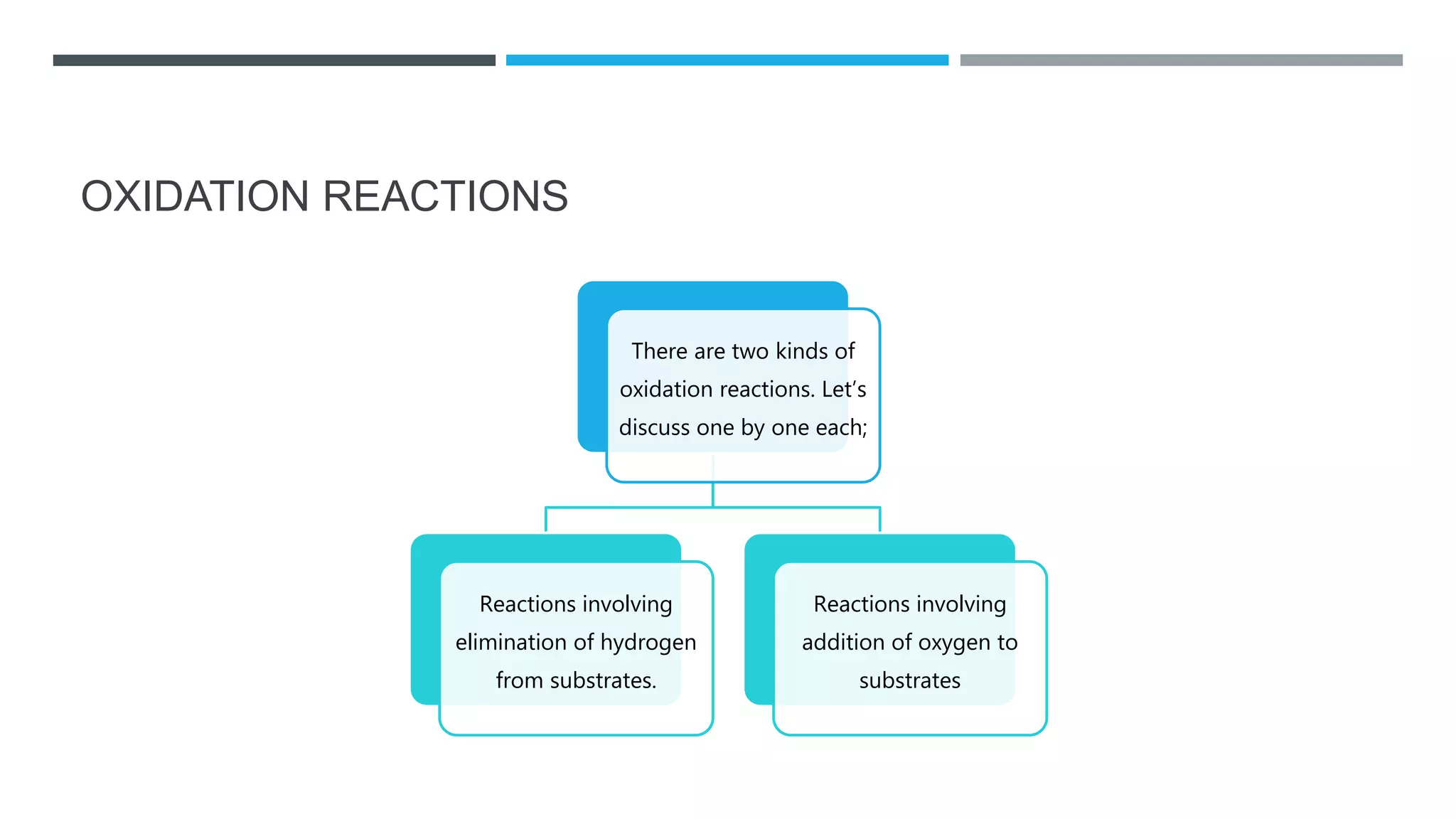
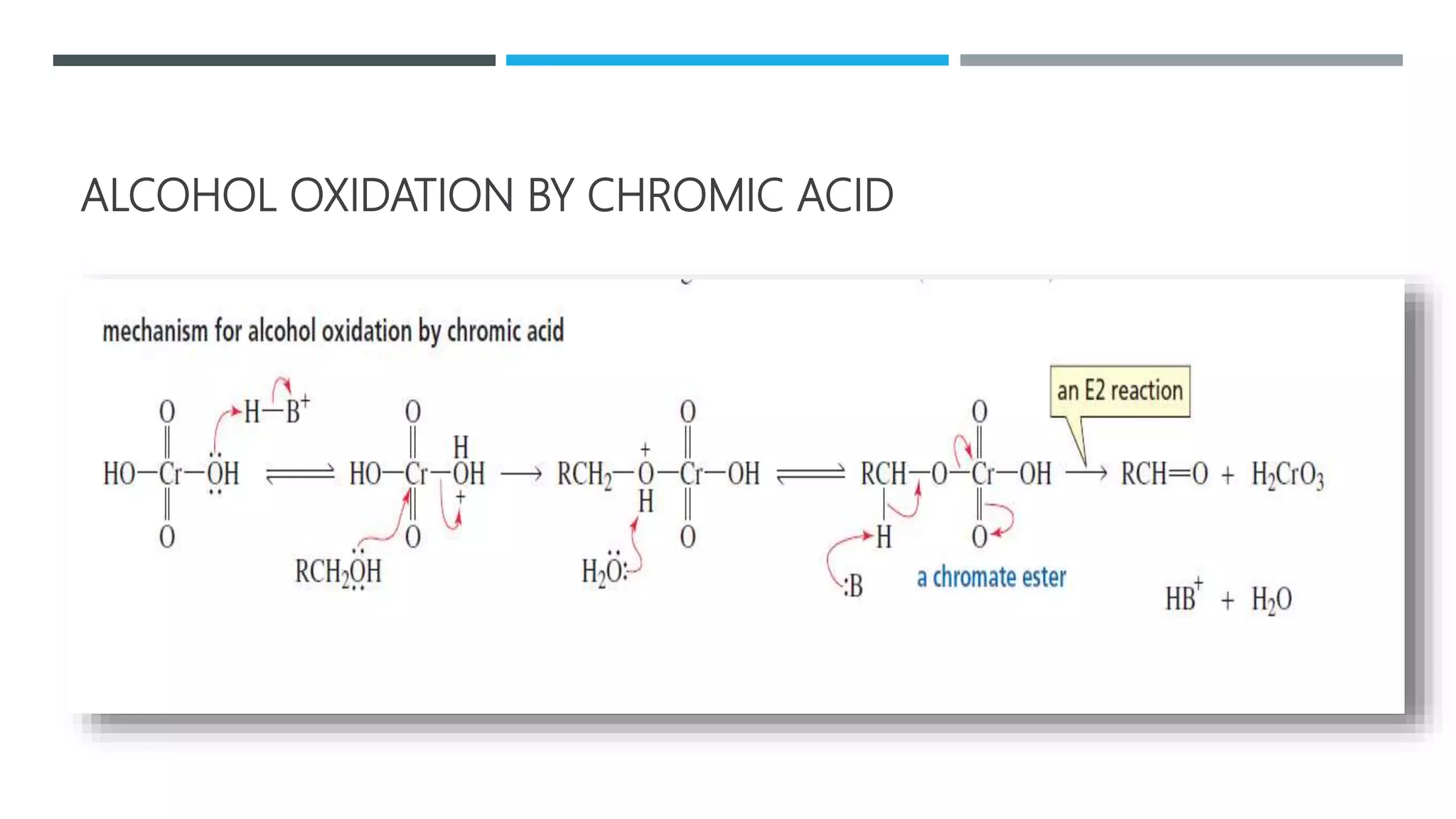
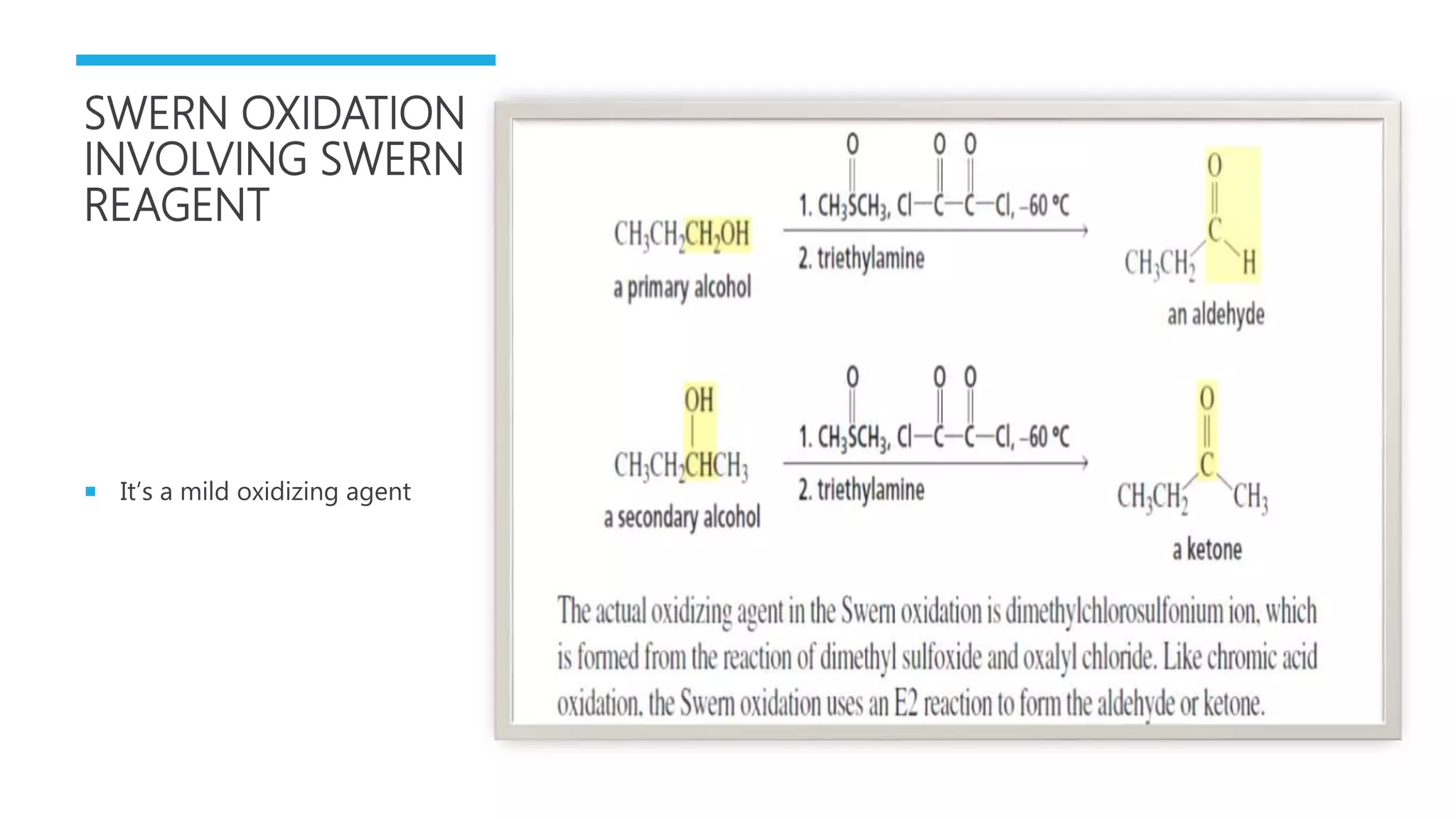
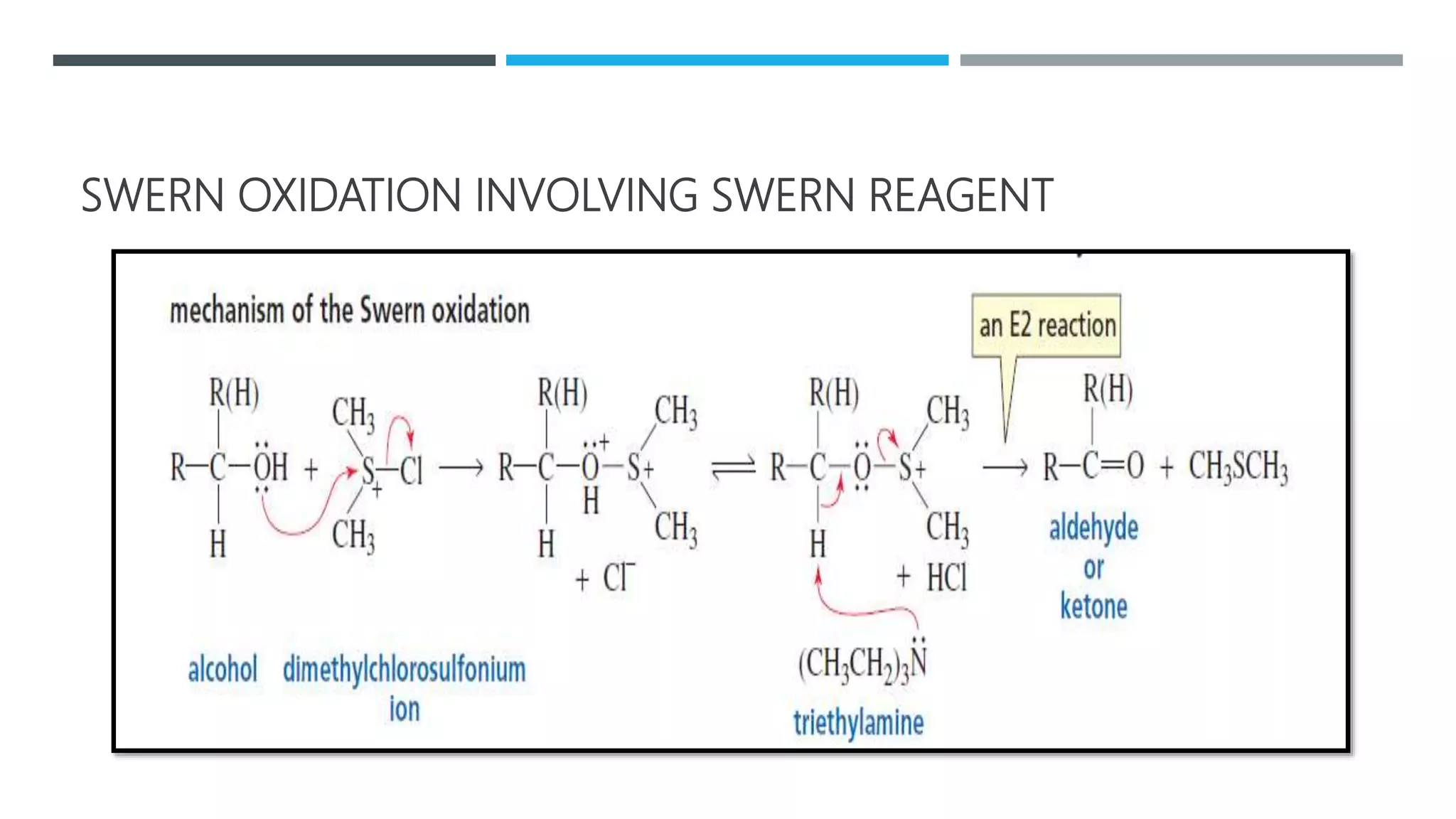
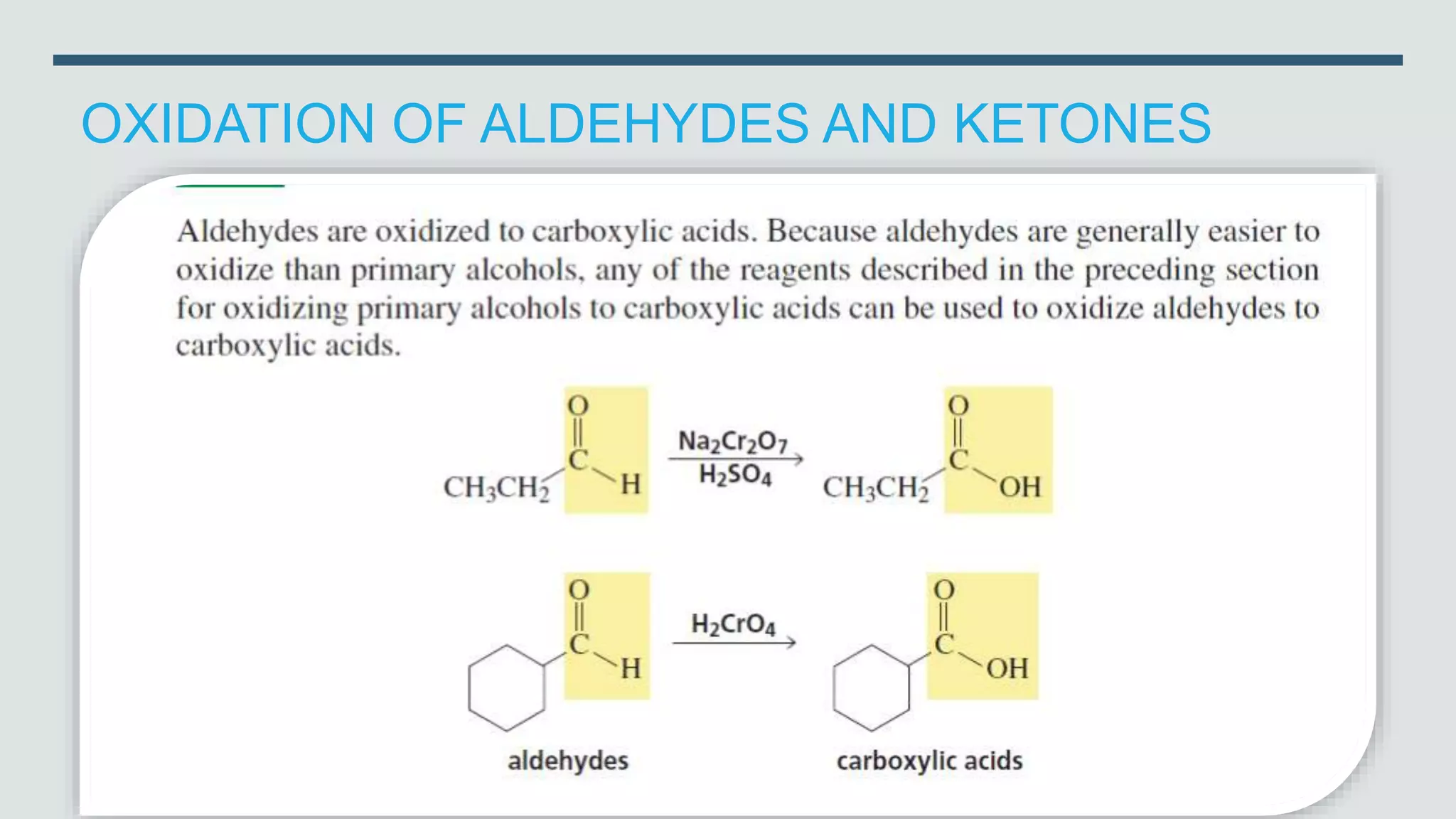
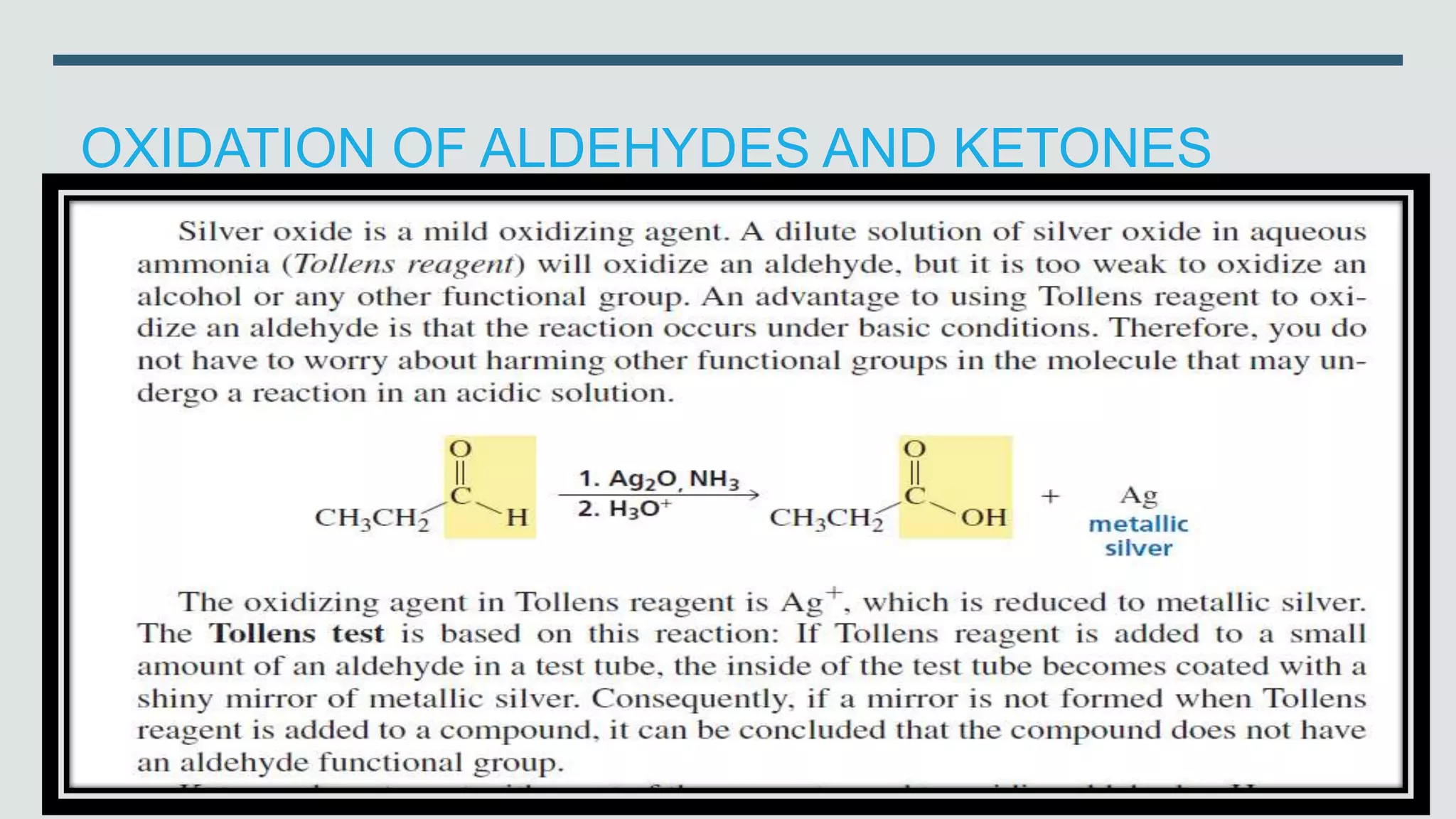
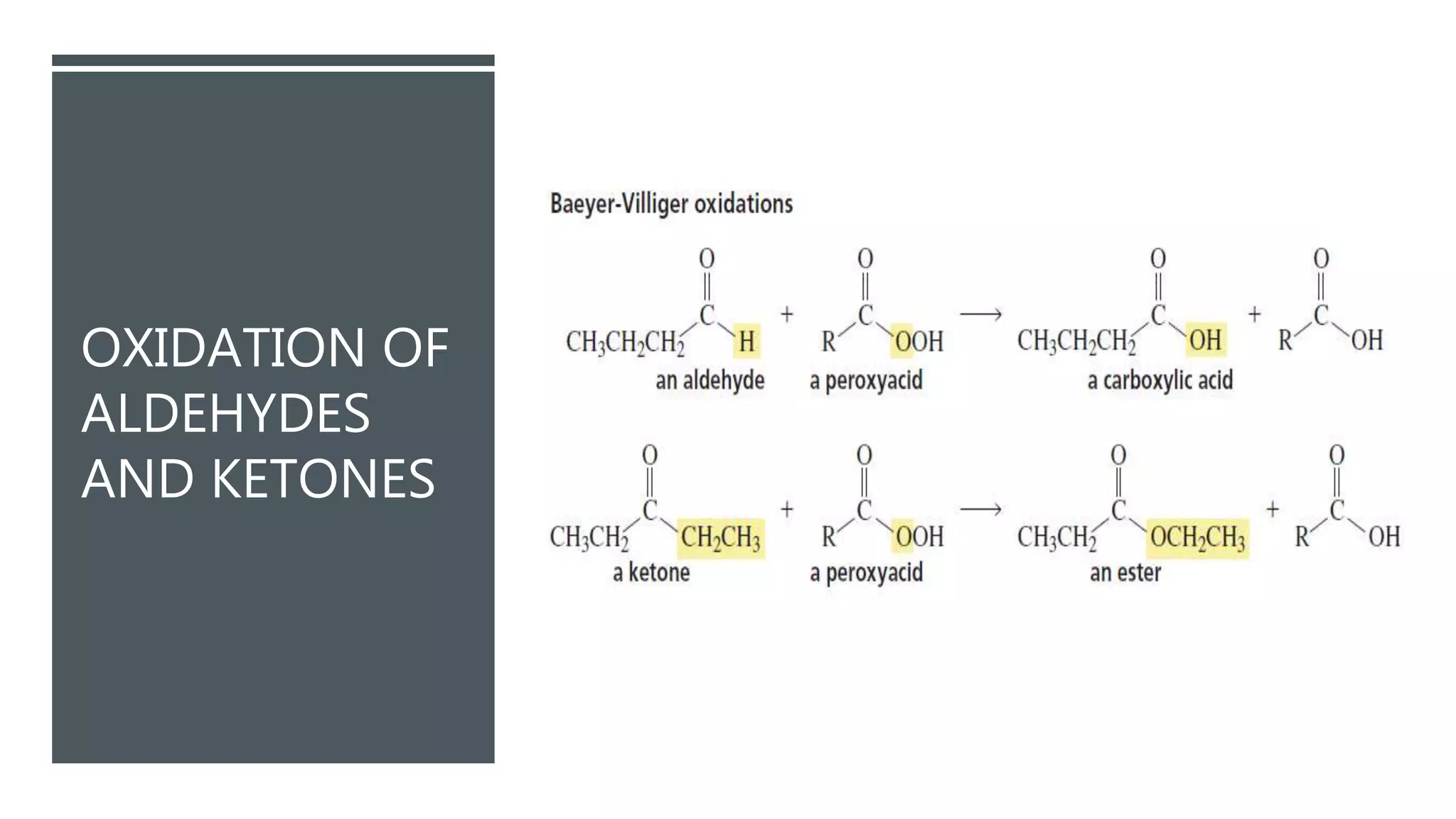
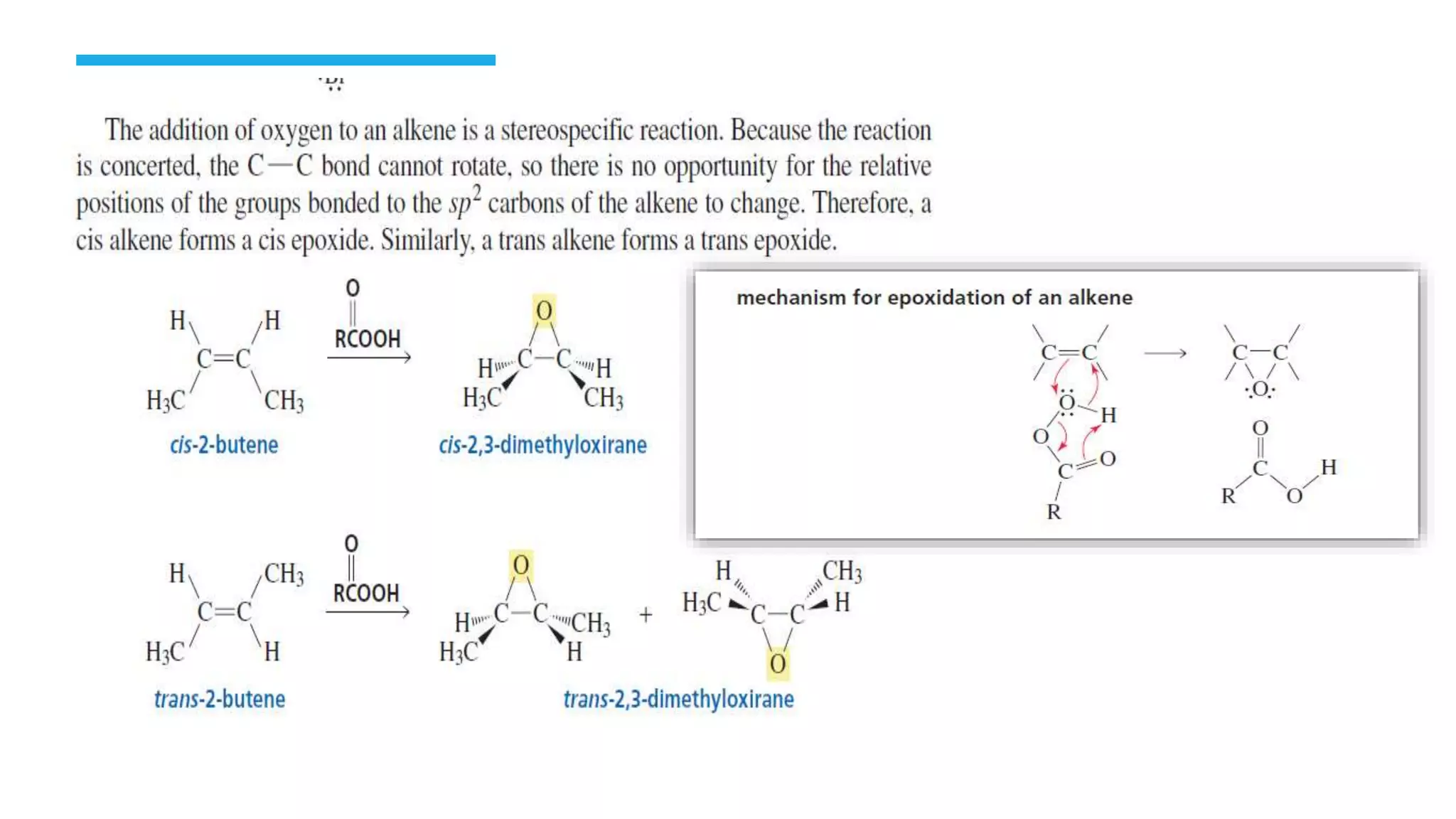
The document defines oxidation as the loss of electrons and reduction as the gain of electrons, collectively known as redox reactions. It discusses various oxidation reactions, primarily focusing on the oxidation of alcohols, aldehydes, ketones, and alkenes, along with several specific reagents and methods used in these processes. The document also highlights the roles of oxidizing and reducing agents in chemical reactions.