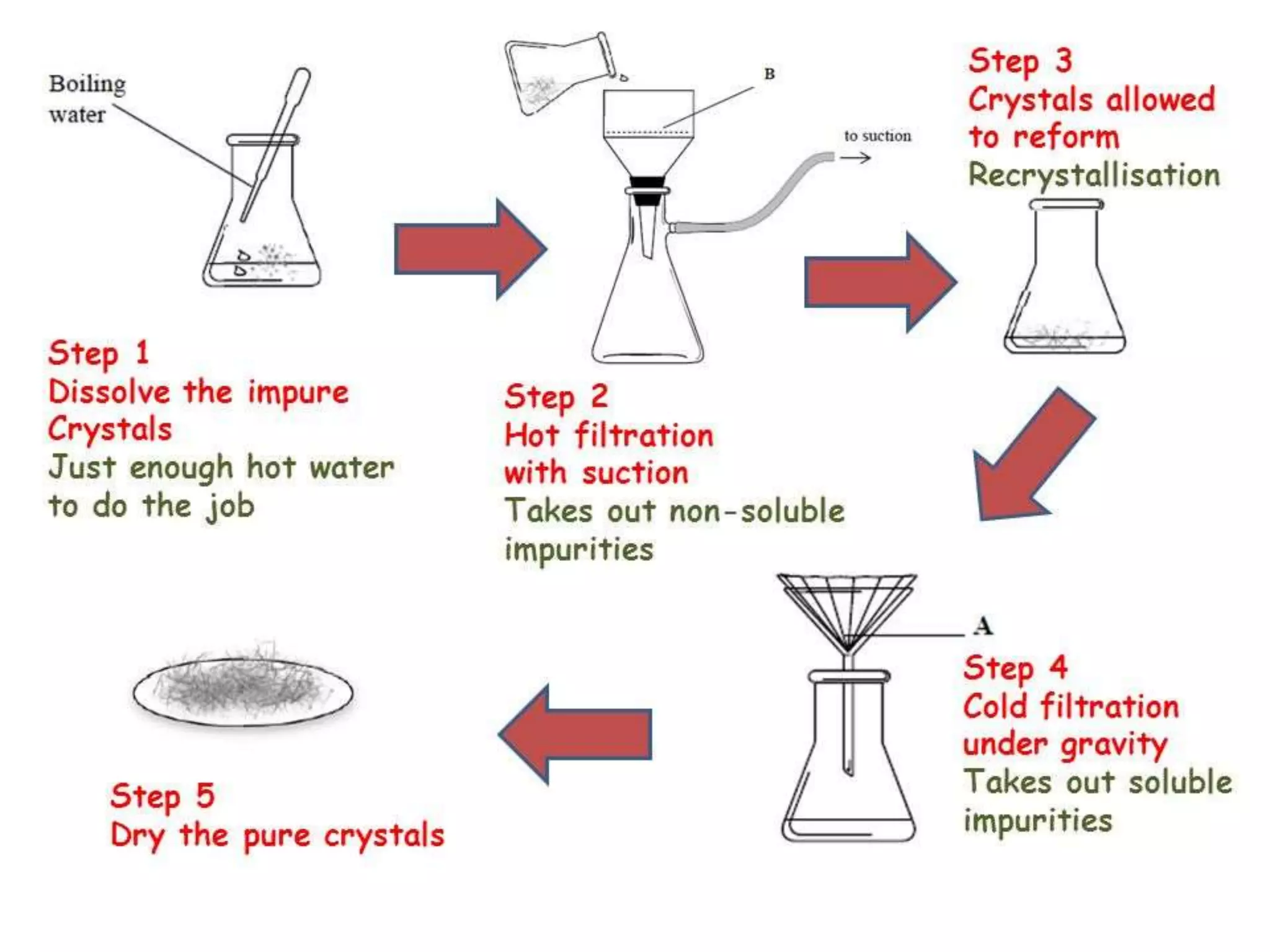
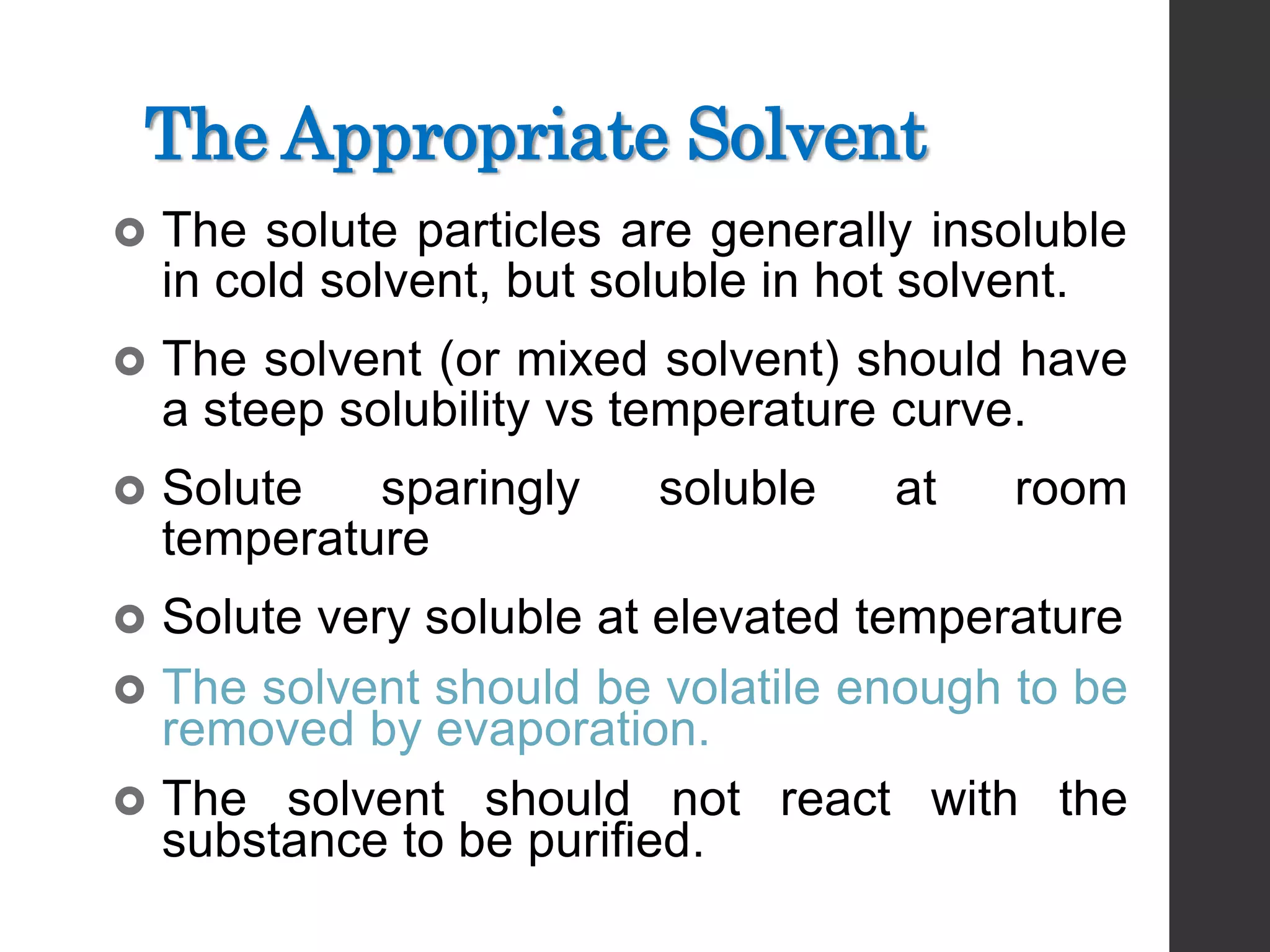
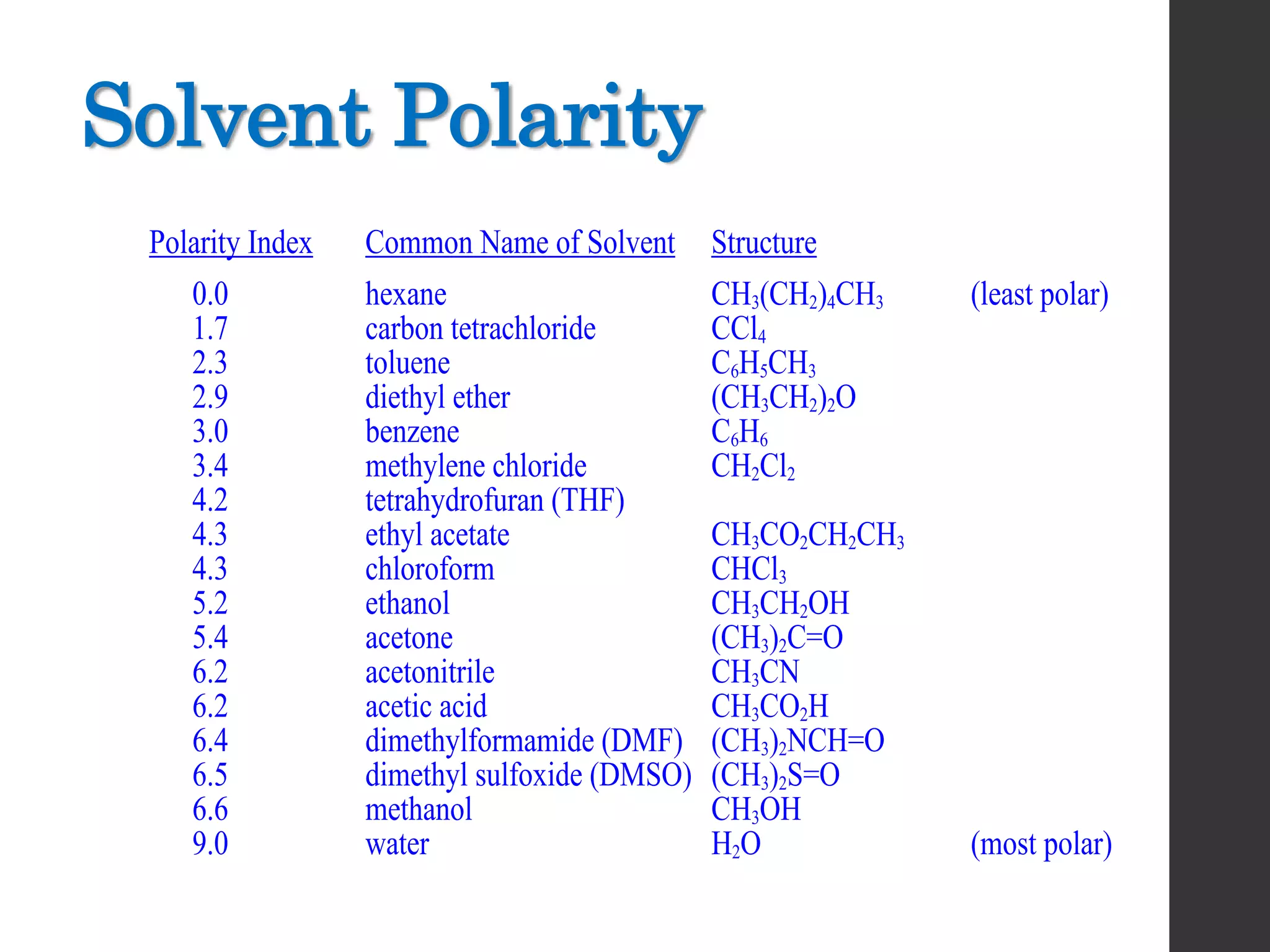
This document discusses the process of recrystallization for purifying solid compounds. It begins by stating the aims and providing references. It then explains that solid compounds produced in the lab usually need purification, and that recrystallization followed by vacuum filtration is commonly used. The key steps of recrystallization are to dissolve the compound in a minimum volume of hot solvent, allow it to crystallize upon cooling, and then isolate the crystals by vacuum filtration. Factors like choosing an appropriate solvent and filtration methods are also outlined.