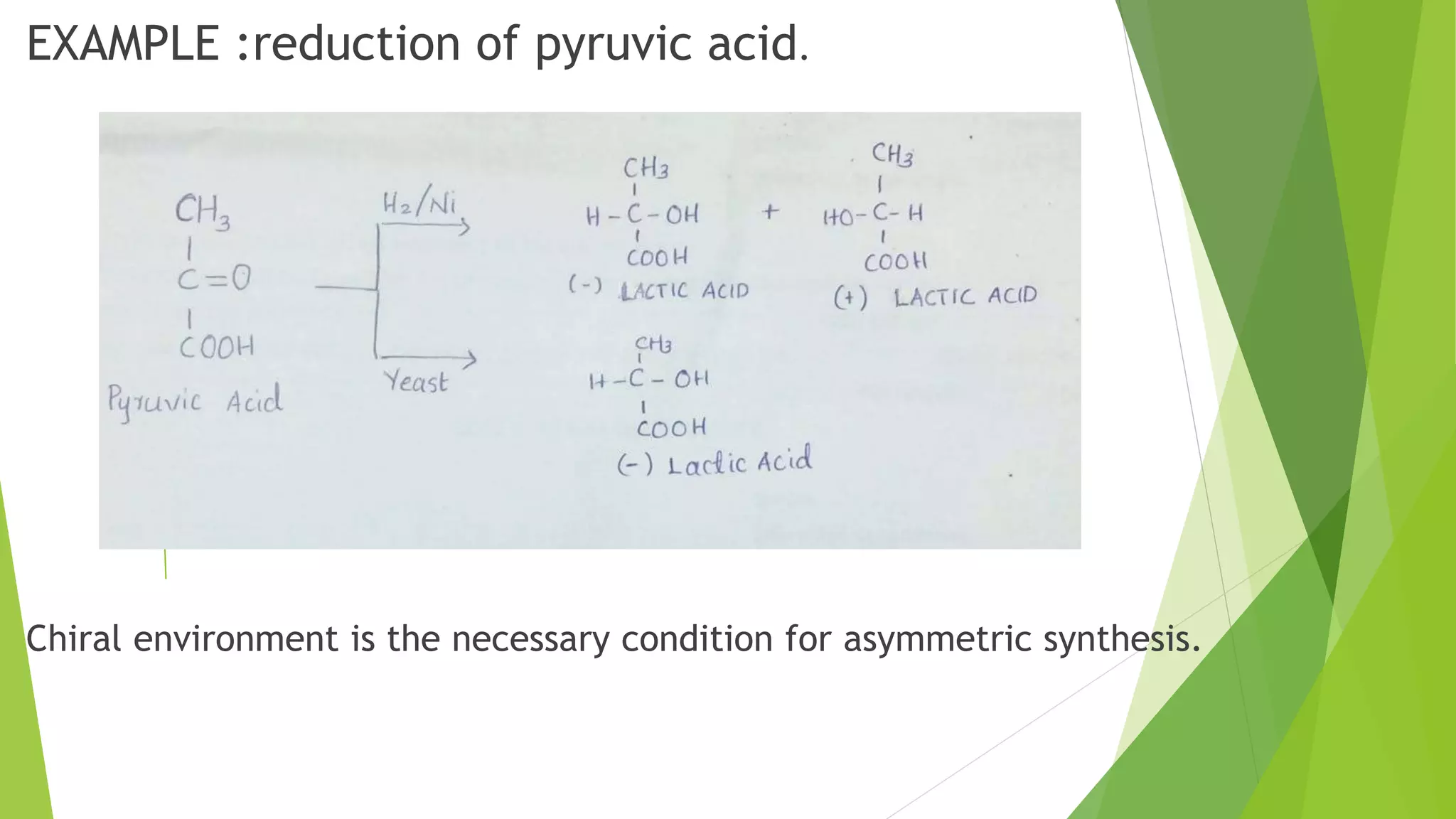
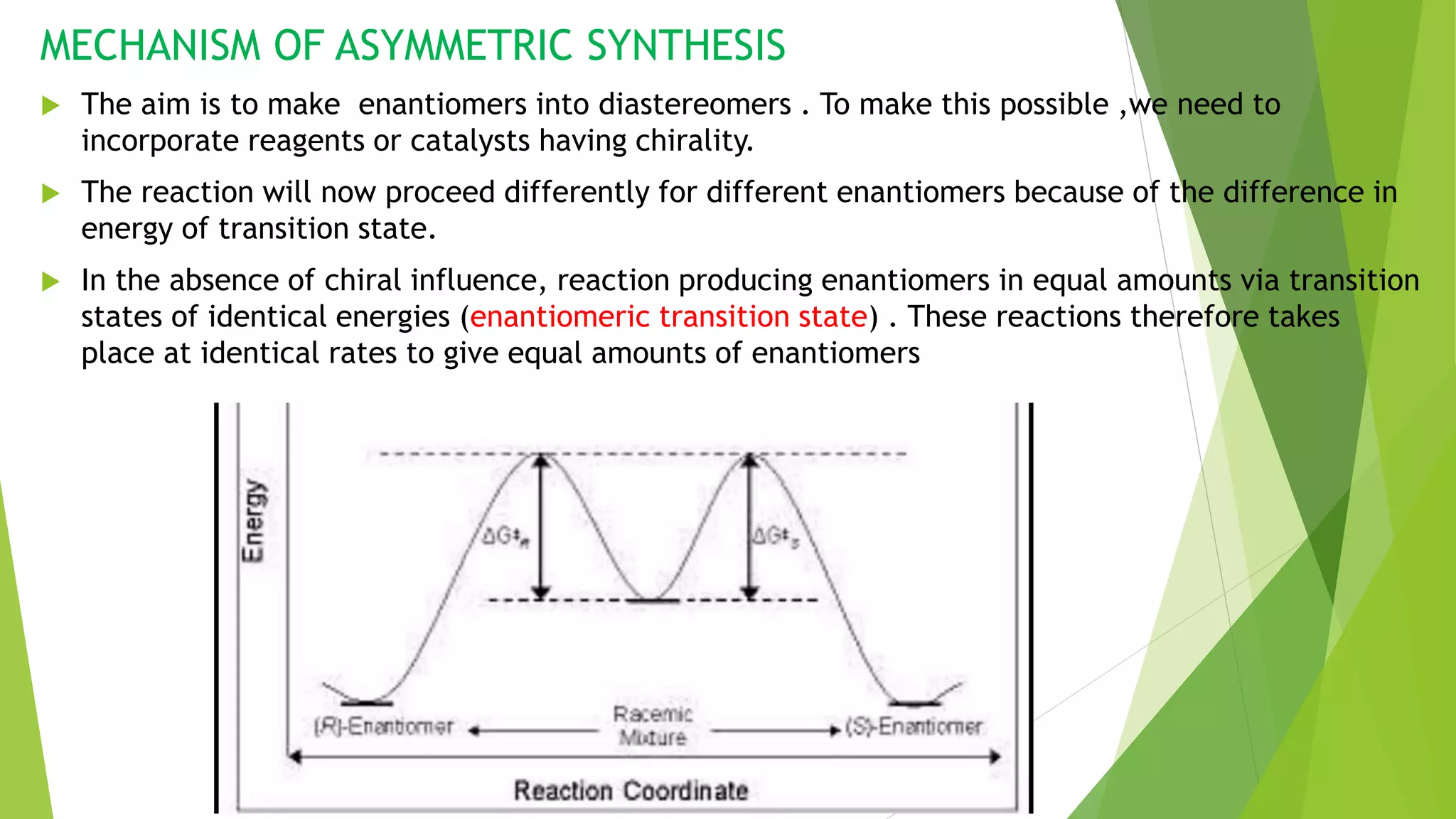
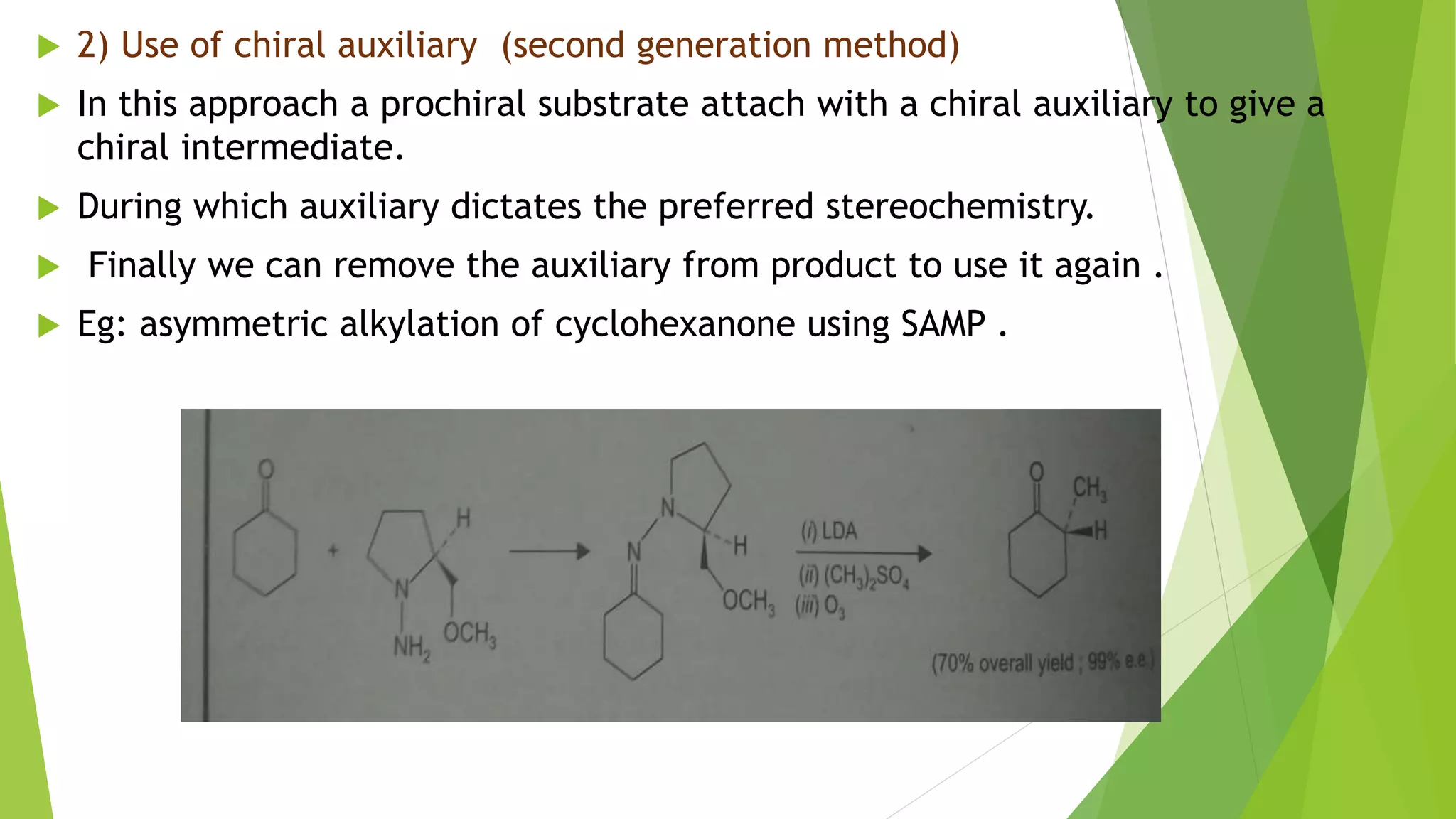
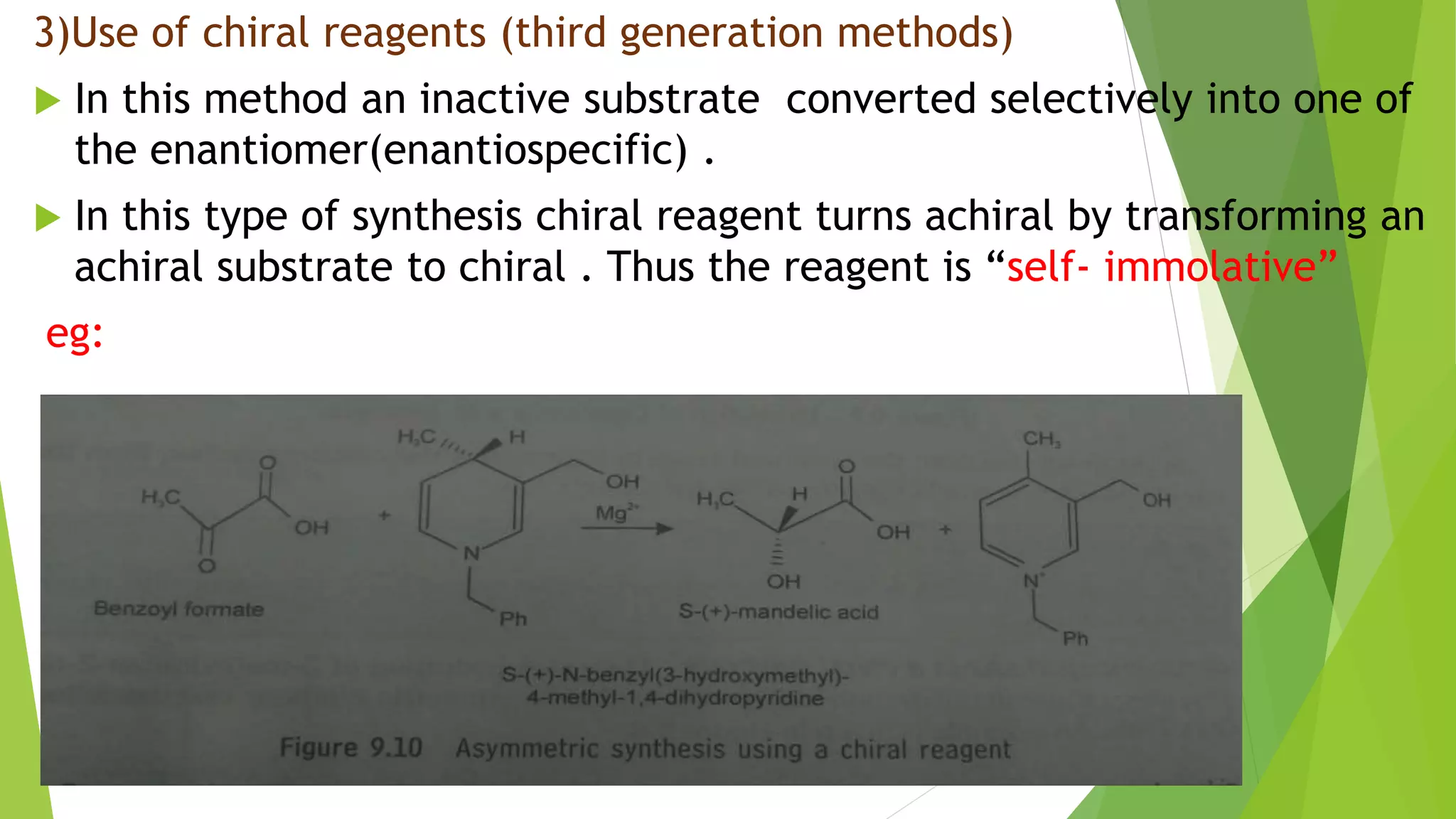
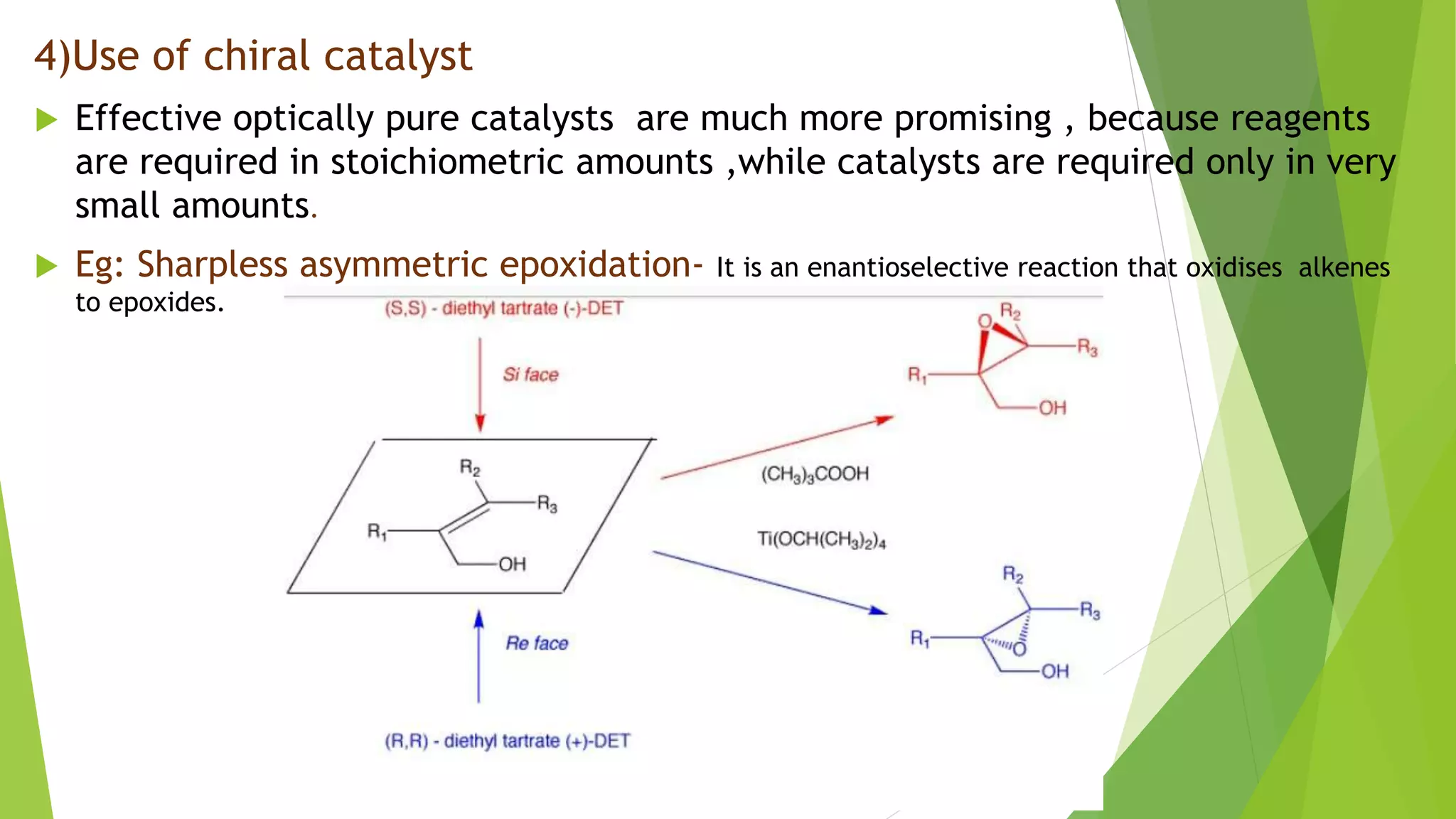
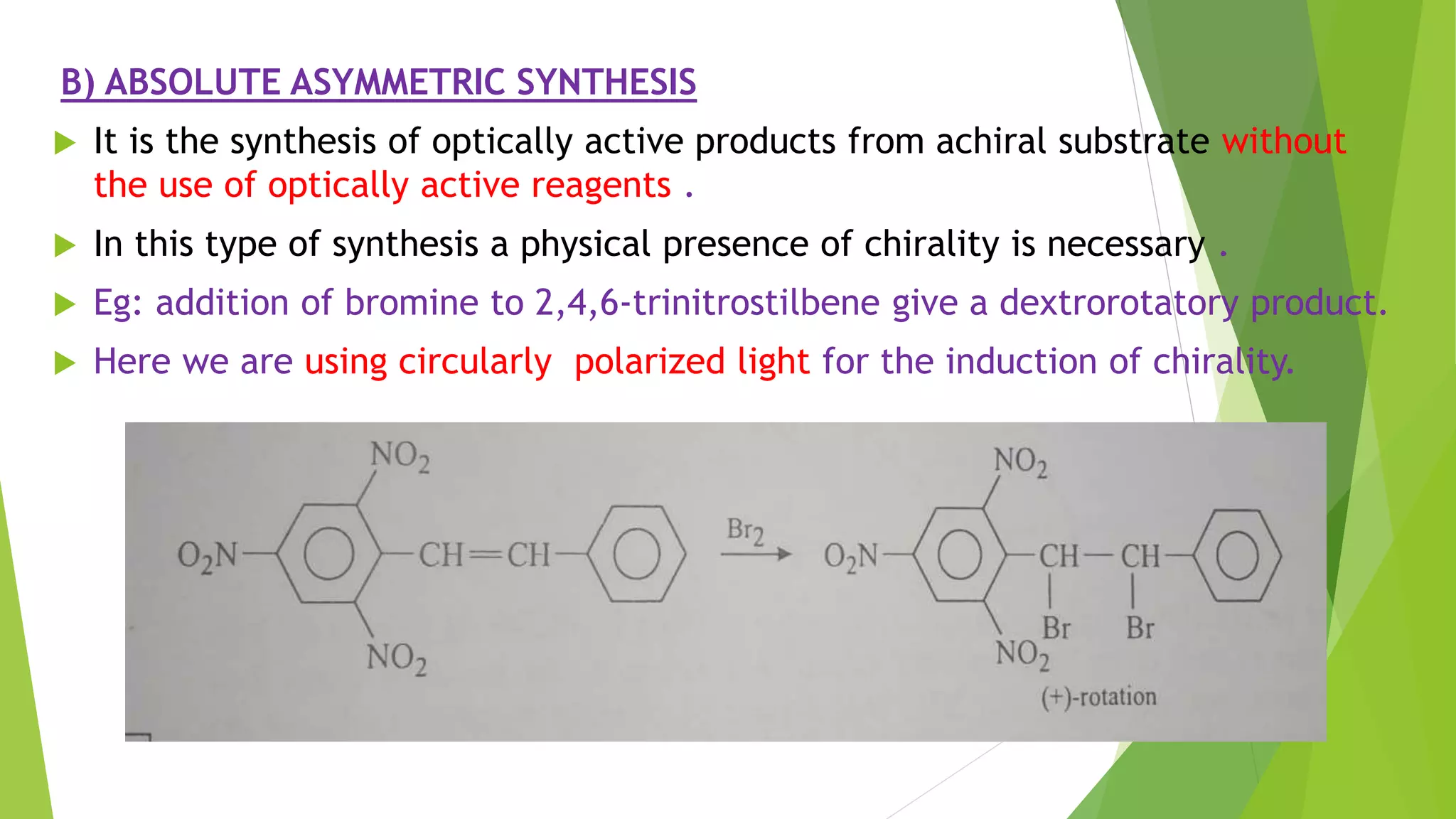
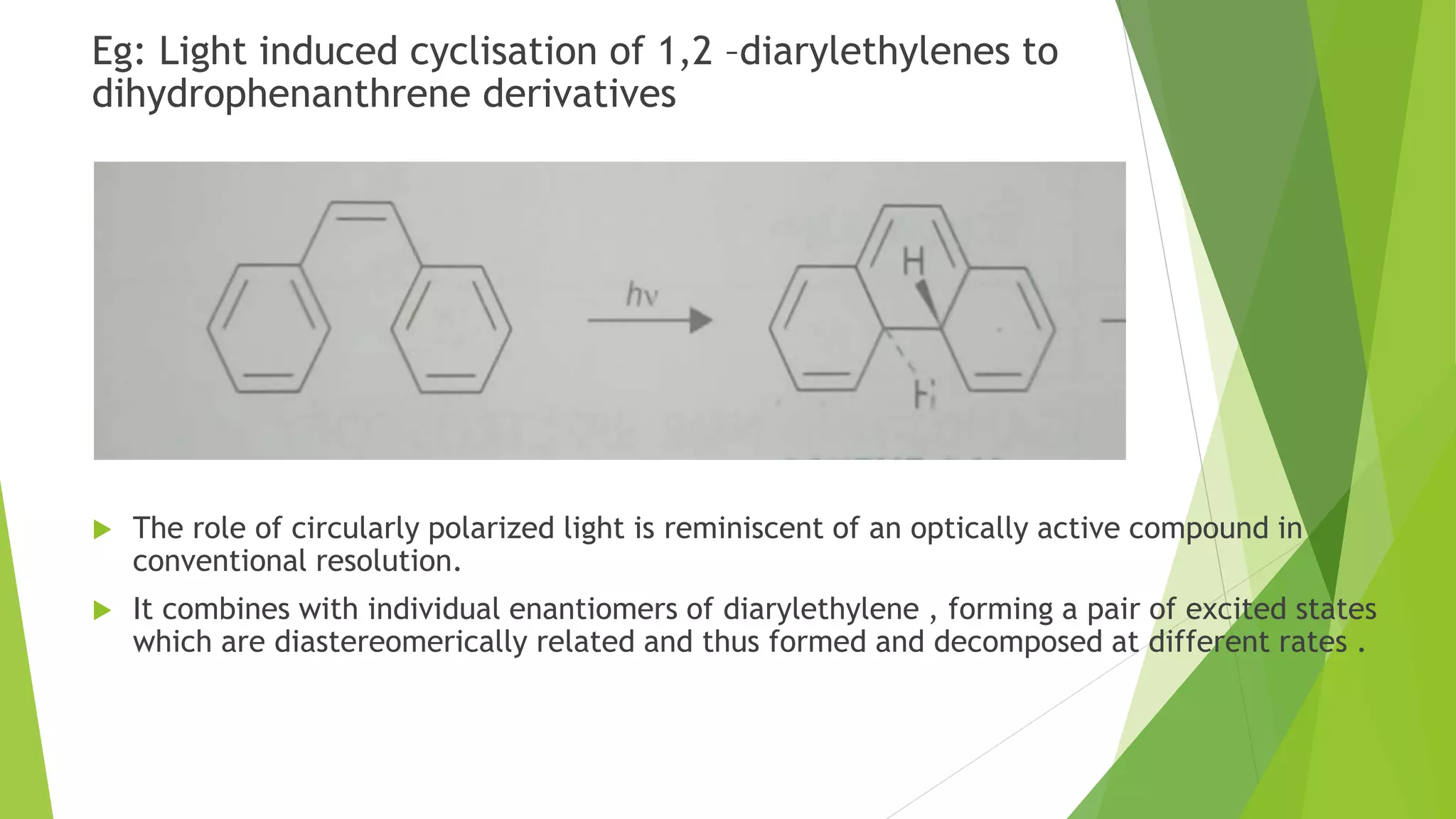
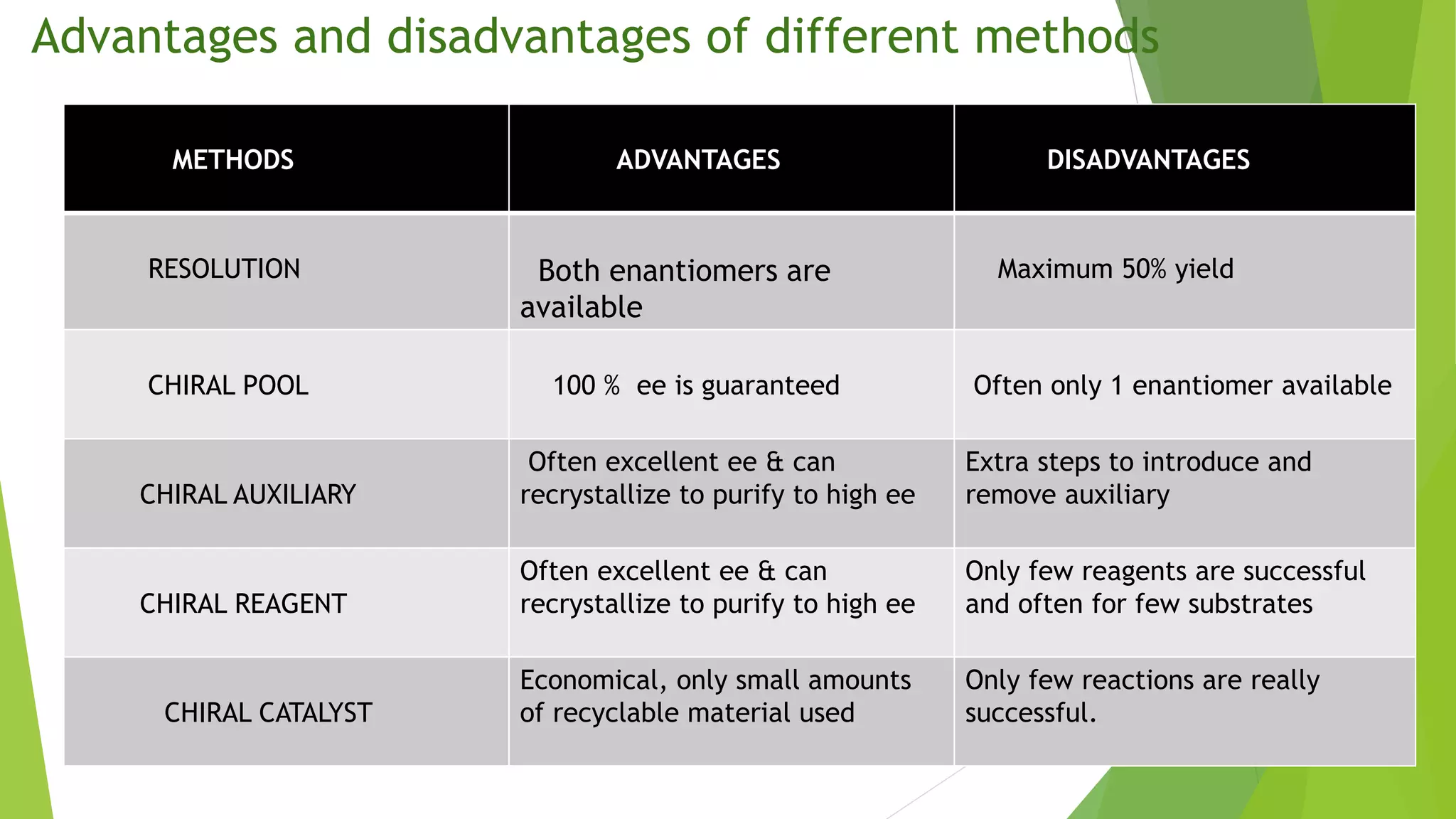
This document discusses asymmetric synthesis, which produces unequal amounts of stereoisomers from achiral precursors. It can be enantioselective or diastereoselective. There are two types: partial asymmetric synthesis, which forms a new chiral center from an achiral precursor using a chiral substrate, auxiliary, reagent, or catalyst; and absolute asymmetric synthesis, which uses no chiral precursors but instead relies on physical chirality like circularly polarized light. Common approaches include using a chiral pool substrate, chiral auxiliary, chiral reagent, or chiral catalyst. The mechanisms and examples of various methods are explained in detail.