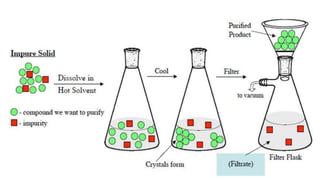
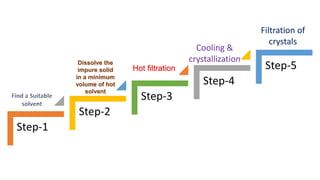
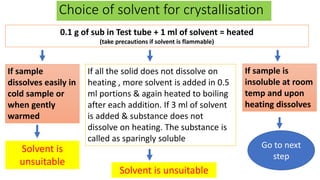
Recrystallization is a technique used to purify solids based on differences in their solubility, involving dissolving the impure solid in a hot solvent, filtering to remove insoluble materials, and obtaining pure crystals during controlled cooling as the solvent crystallizes out of solution. The process works best when an appropriate solvent is selected that dissolves the compound at high temperatures but causes it to crystallize upon cooling, allowing purification through multiple recrystallization attempts if needed.













![? Questions
1] What happens if you add too much solvent
during recrystallization?
2]During filtration, why is it important to only
wash your solid with ice-cold solvent?
3] Why should you fold and put creases in your
filter paper prior to filtration?](https://image.slidesharecdn.com/recrystallisation-200709091319/85/Recrystallisation-17-320.jpg)
![References:
1] Vogel's textbook of quantitative chemical analysis. – 5th
ed.
2] https://slideplayer.com/slide/7629283/](https://image.slidesharecdn.com/recrystallisation-200709091319/85/Recrystallisation-18-320.jpg)