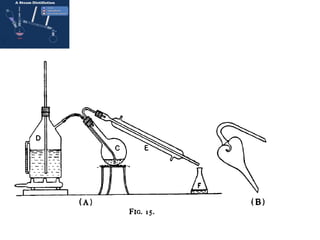
Steam distillation is a distillation technique used to purify temperature-sensitive organic compounds. It involves boiling a mixture using steam instead of direct heat, allowing distillation at lower temperatures that avoid decomposition. The steam distillation process separates compounds based on differences in volatility between the components. The vapors produced are condensed, separating into an aqueous layer containing water and an organic layer containing the purified compound. Steam distillation is commonly used to extract essential oils from plants for perfumes and other applications.