The document discusses local anesthetics (LA), including:
- Their mechanism of action in blocking sodium channels to inhibit nerve conduction and sensation of pain.
- Types include infiltration, nerve block, spinal, epidural, and caudal anesthesia.
- Common LA drugs are procaine, lidocaine, tetracaine, and bupivacaine. Cocaine was the first LA discovered.
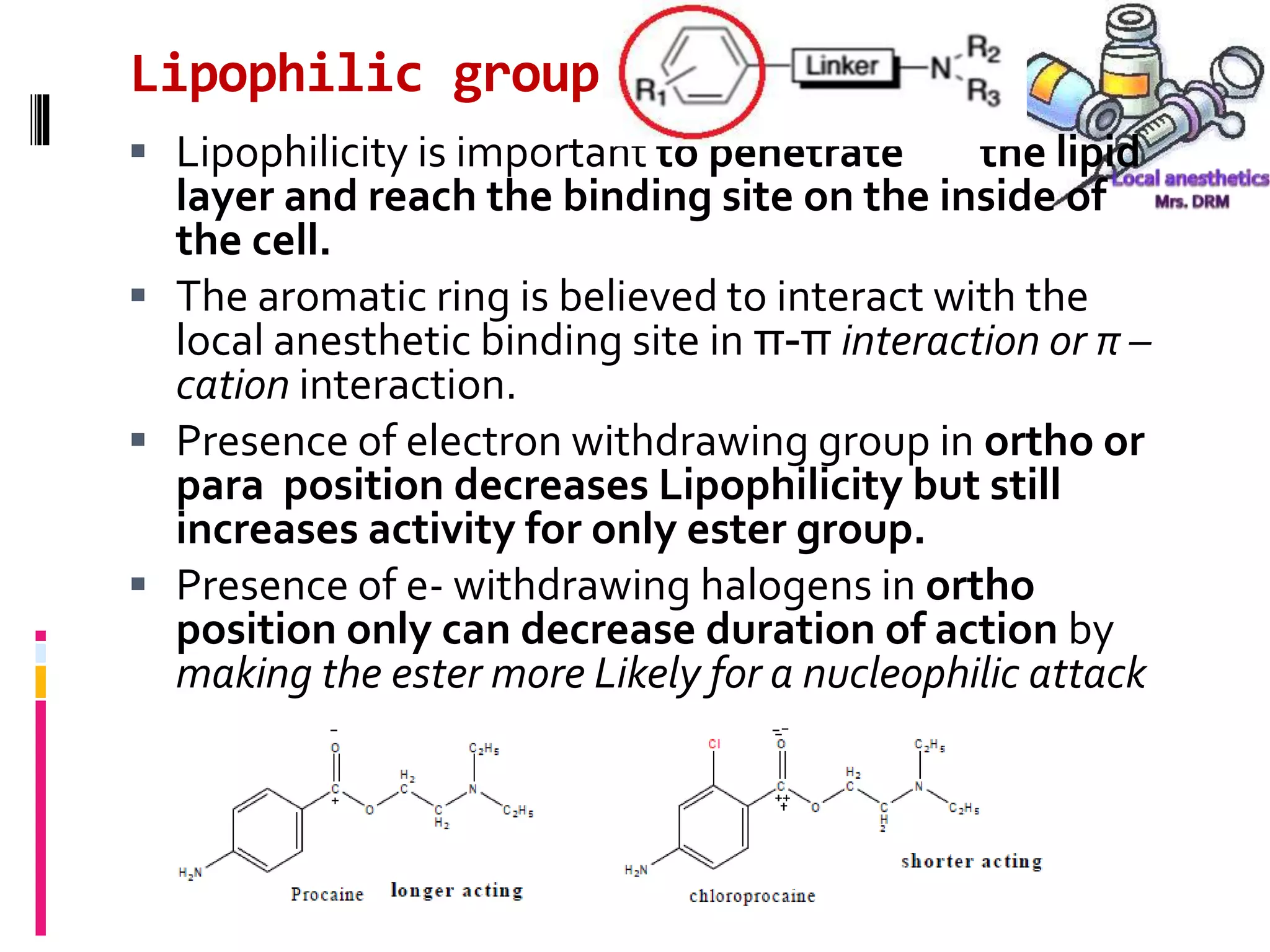
- LA chemistry aims to balance lipid solubility for potency versus ionization for reduced toxicity.