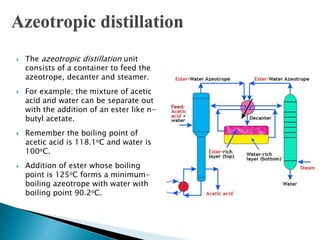
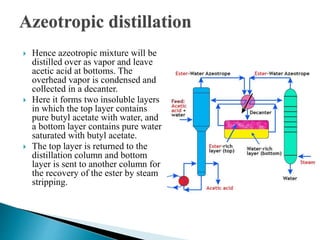
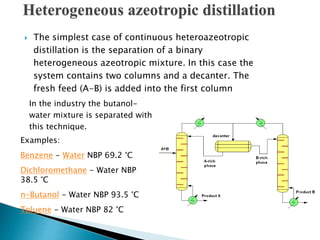
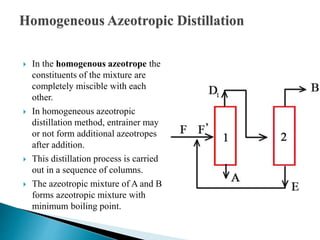
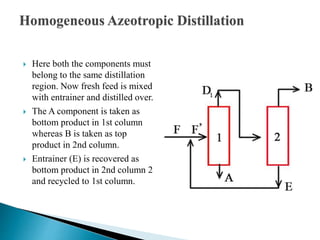
The document discusses azeotropic and steam distillation. It defines azeotropes as mixtures that have the same composition in both the liquid and vapor phases, preventing separation through simple distillation. There are two types: minimum boiling and maximum boiling azeotropes. Methods to separate azeotropes include pressure swing distillation, azeotropic distillation using an entrainer, and steam distillation for heat-sensitive compounds. Azeotropic distillation works by forming a new low-boiling azeotrope with the entrainer, then separating the components in a decanter. Steam distillation uses water vapor to carry compounds over at lower temperatures than simple distillation