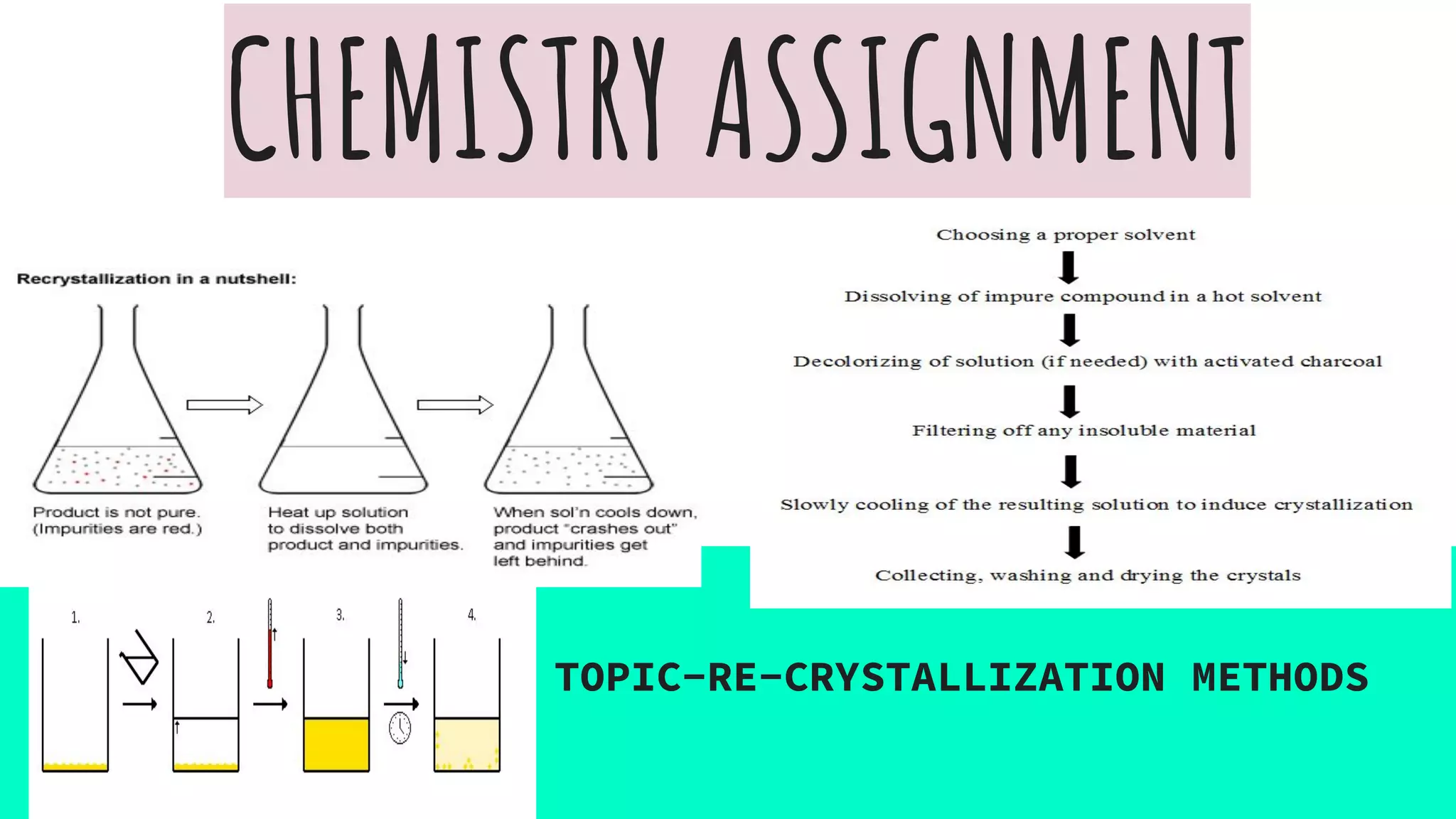
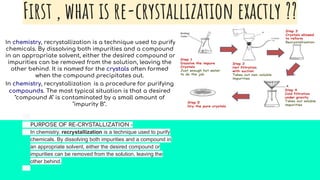
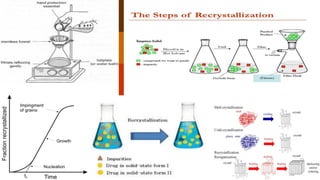
Recrystallization is a purification technique in chemistry that involves dissolving both a compound and its impurities in a suitable solvent, allowing for the removal of unwanted substances and the formation of pure crystals. The principle relies on the increased solubility of solutes in solvents at elevated temperatures, leading to crystallization upon cooling. Selecting the right solvent is crucial, as it should dissolve the impurities at room temperature while allowing the desired compound to precipitate when cooled.