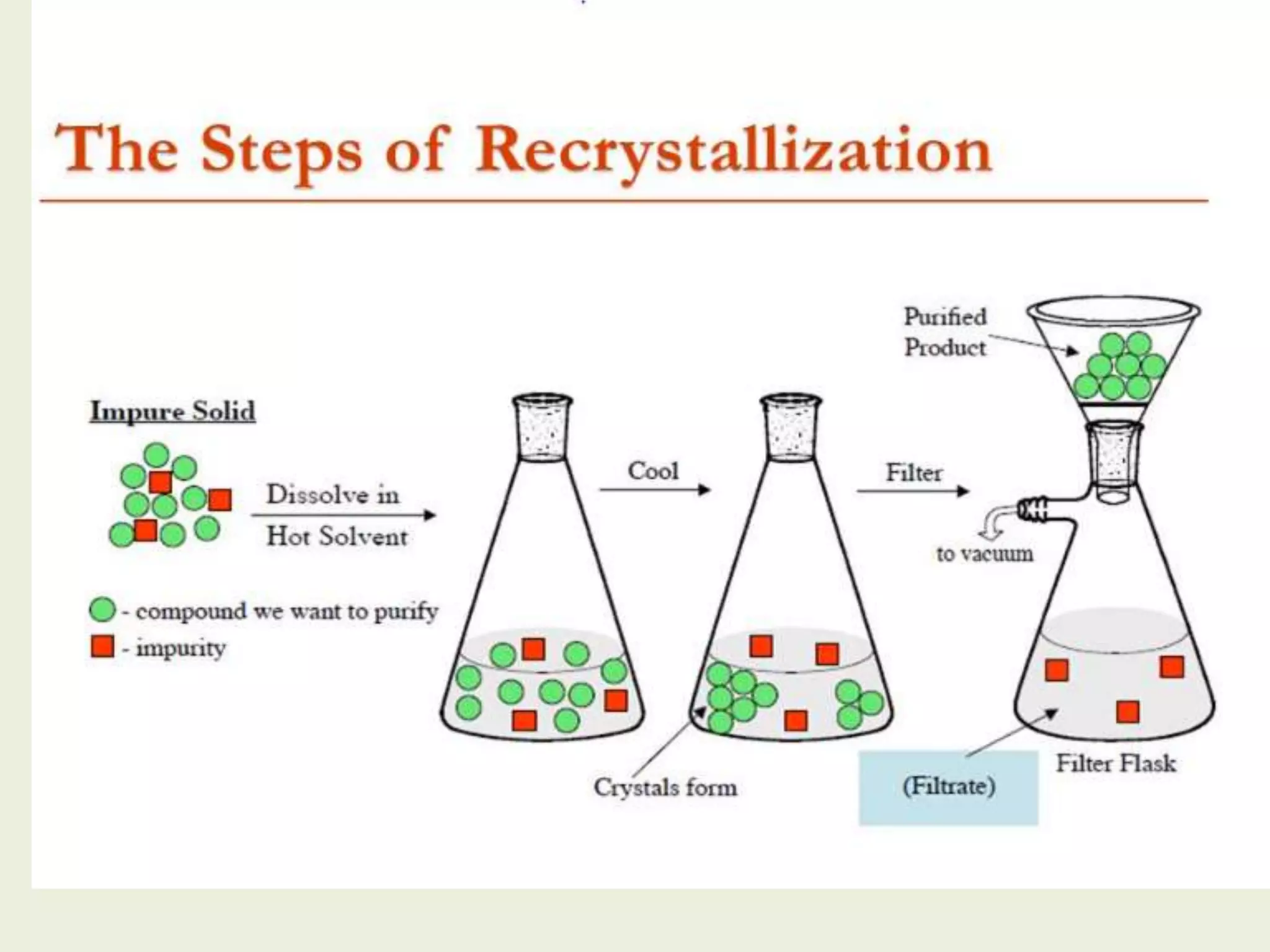
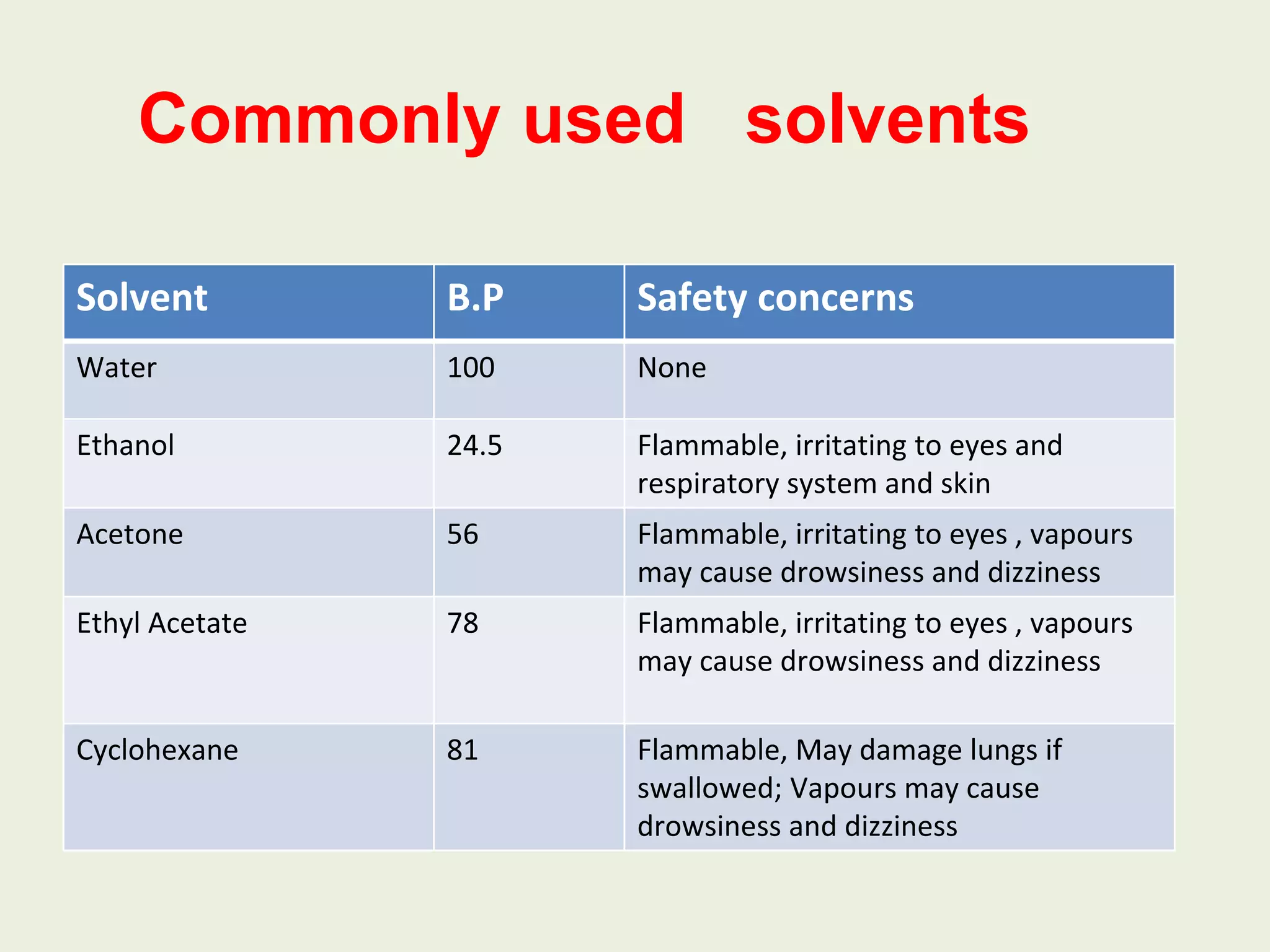
Recrystallization is a technique used to purify impure solids by dissolving them in a hot solvent and slowly cooling the solution to allow the desired compound to crystallize out. There are different recrystallization methods like single-solvent or multi-solvent. For recrystallization to work, there must be a difference in solubility between the compound and impurities as temperature changes, so organic compounds are typically more soluble in hot solvents than cold solvents. The general procedure involves choosing a solvent, dissolving the impure compound in a minimum amount of hot solvent, filtering to remove impurities, and slowly cooling the solution to obtain purified crystals of the desired compound.