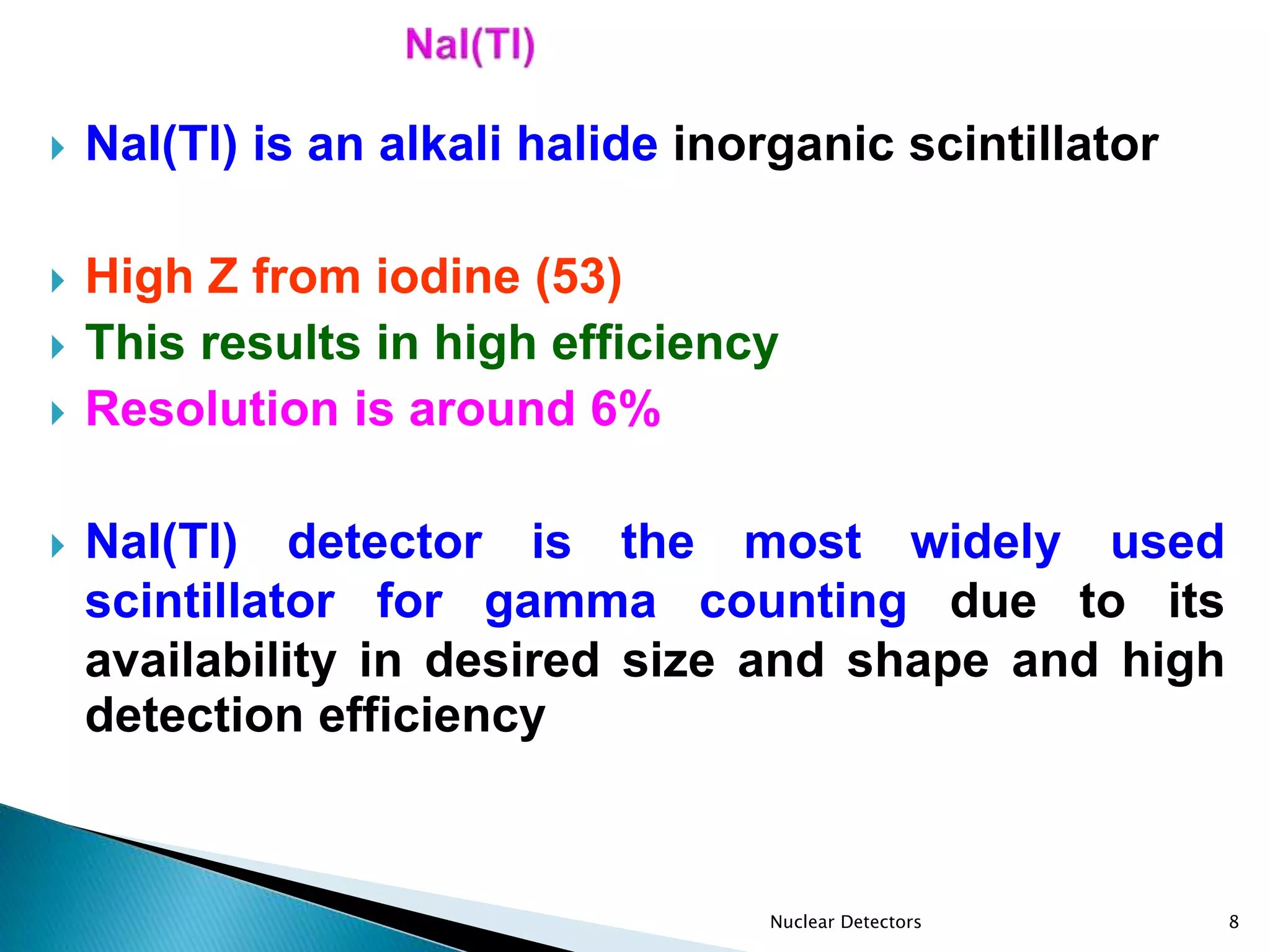
This document discusses scintillation detectors and their properties. Scintillation detectors work by emitting light when exposed to radiation, and this light output can be used to measure incident radiation. The key properties of scintillation detectors are that they must have high scintillation efficiency, light yield proportional to deposited energy, short decay time, transparency to emitted wavelengths, and be able to be made in large sizes and desired shapes. Common inorganic scintillators discussed are NaI(Tl), which is widely used due to its availability and high detection efficiency, and BGO, which has high intrinsic efficiency for high gamma energies.







![ Solvent = Dioxan (1 litre)
Scintillator = PPO (0.7%) (2,5 diphenyl oxazole)
Wavelength shifter = POPOP (0.03%)
(1,4 bis-[2-(5-phenyloxazolyl)]-benzene
To keep Pu in the complex form = TOPO (2%)
Anti quencher = Naphthalene (10%)
Nuclear Detectors 12](https://image.slidesharecdn.com/scintillationdetector-230505045145-fd0cb409/75/Scintillation-Detector-pptx-12-2048.jpg)