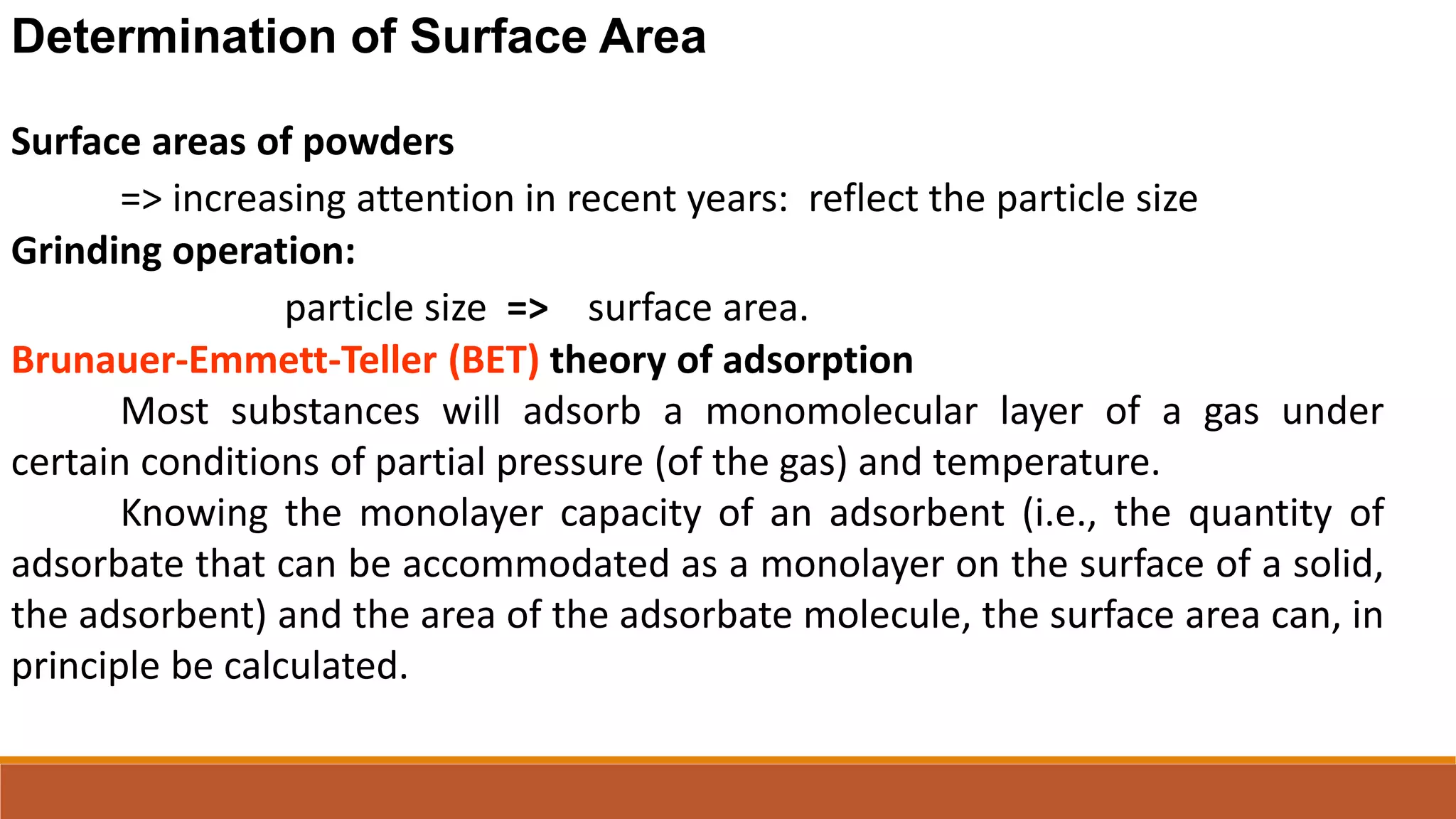
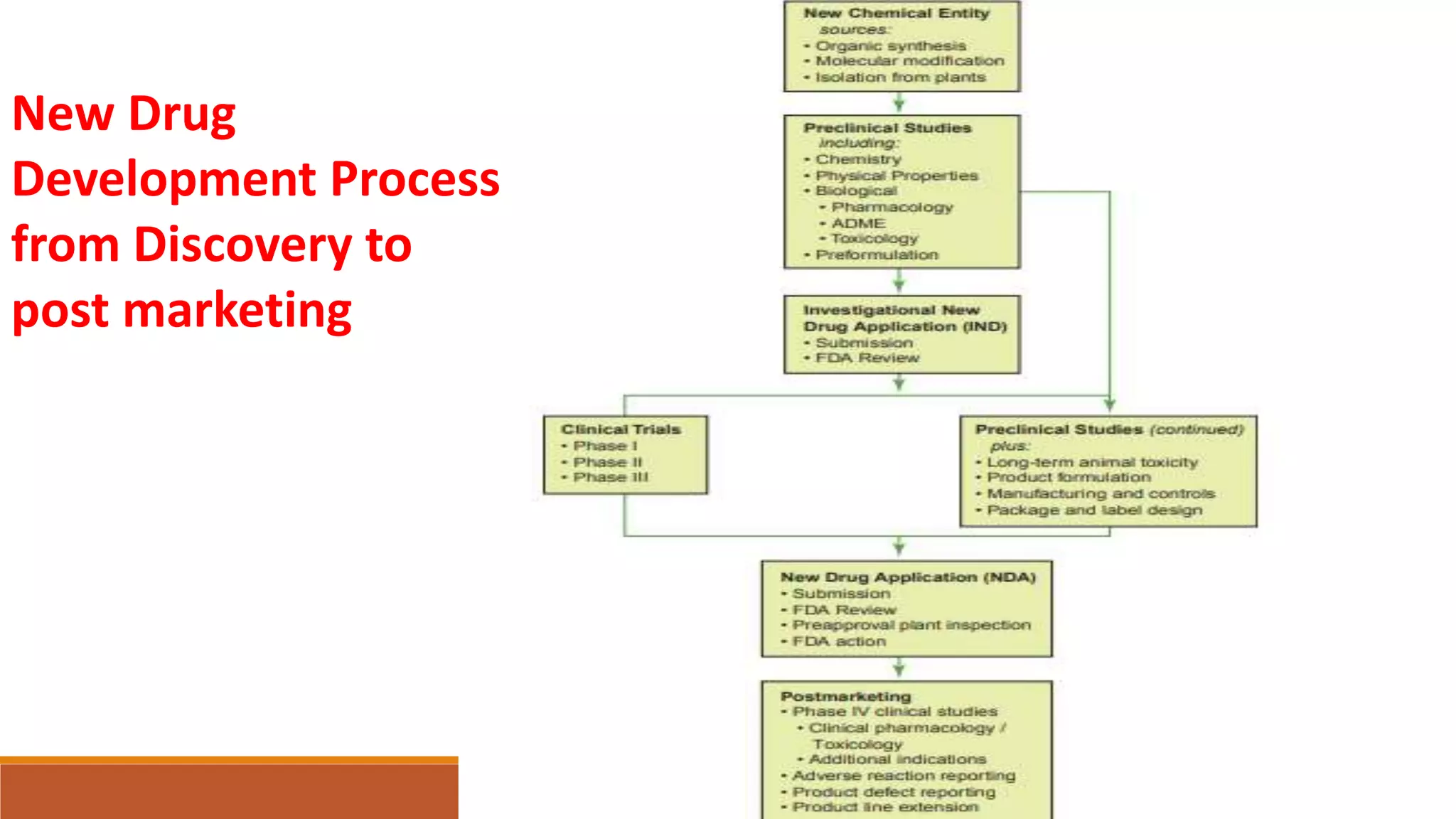
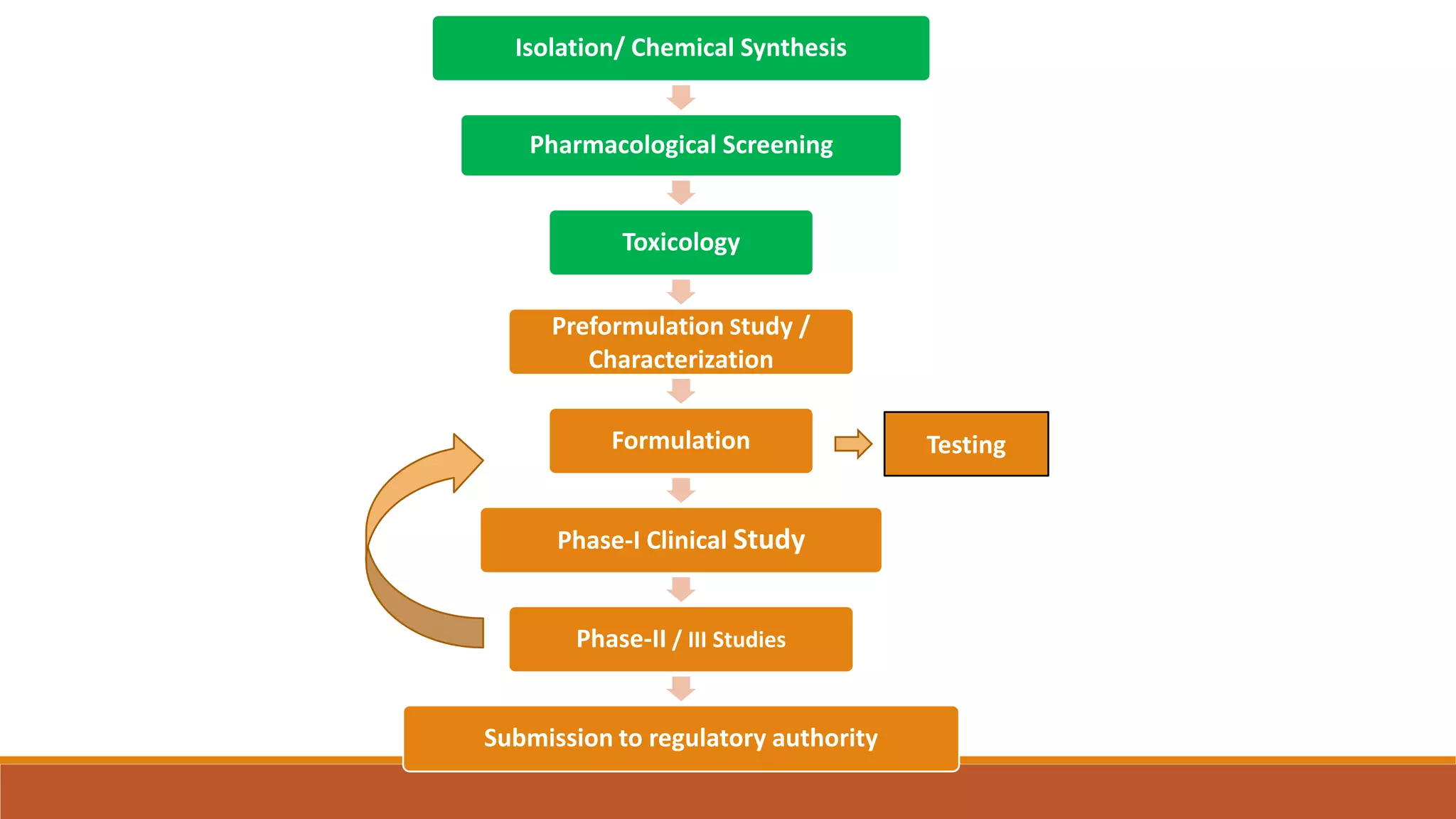
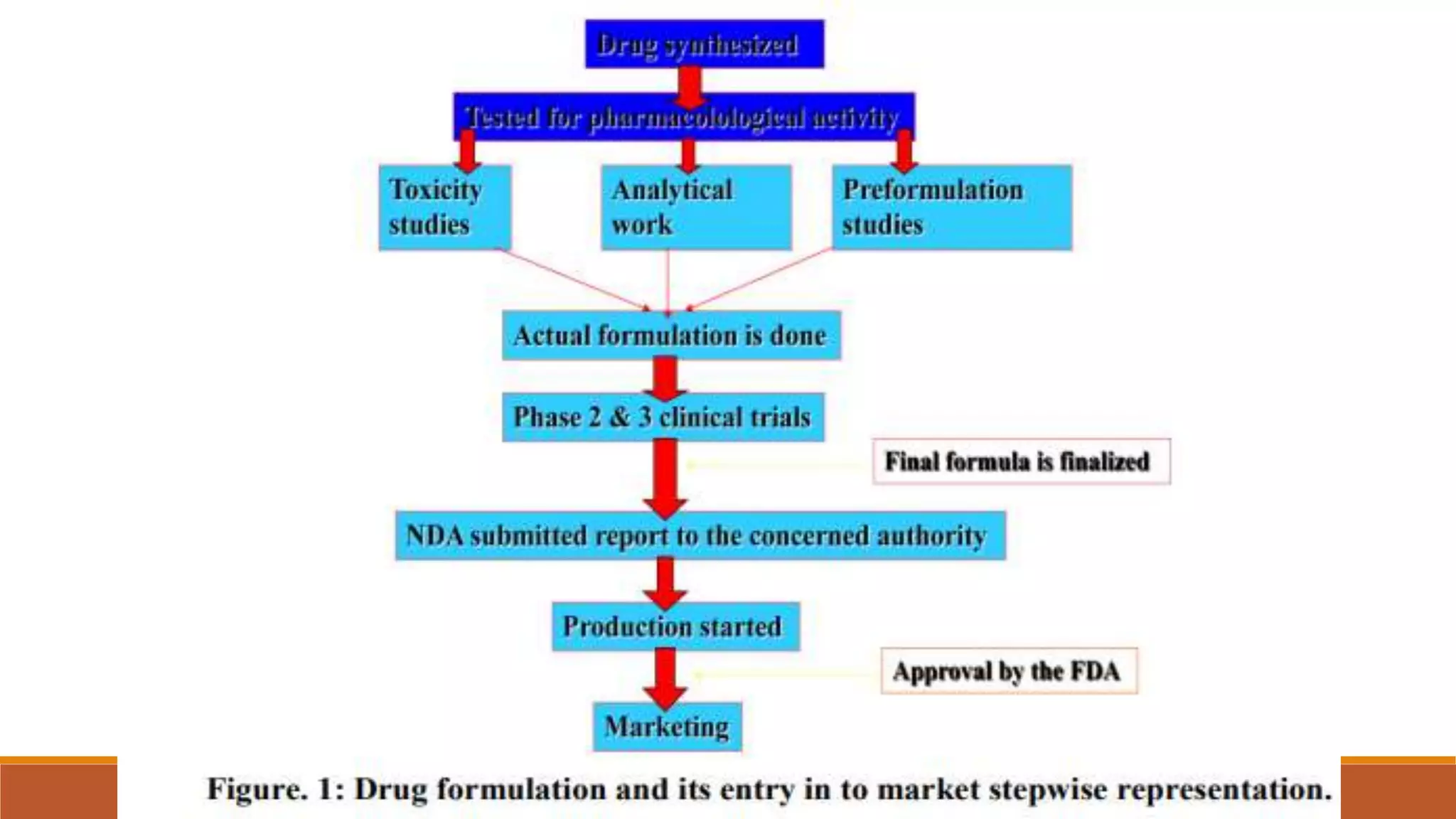
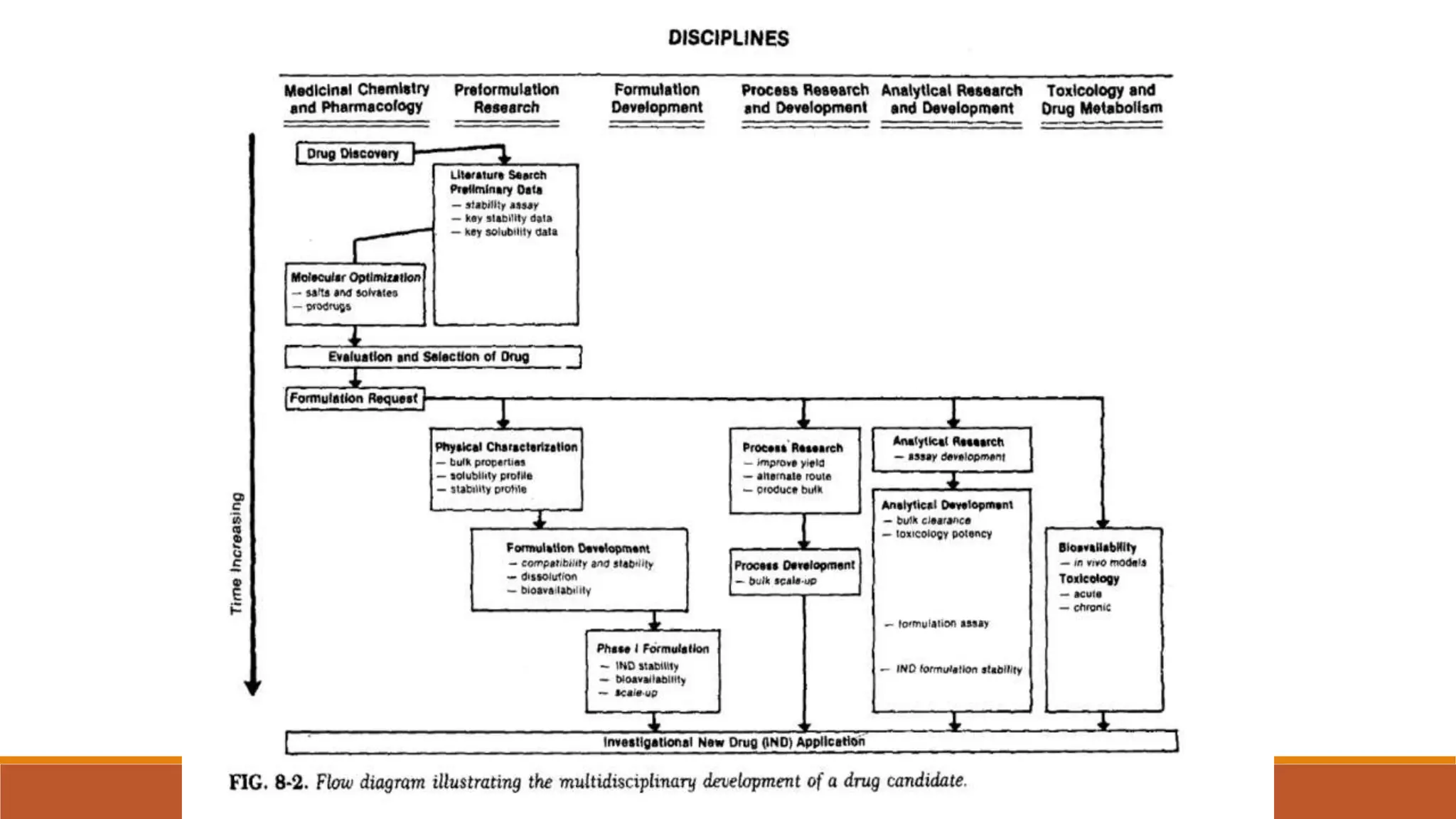
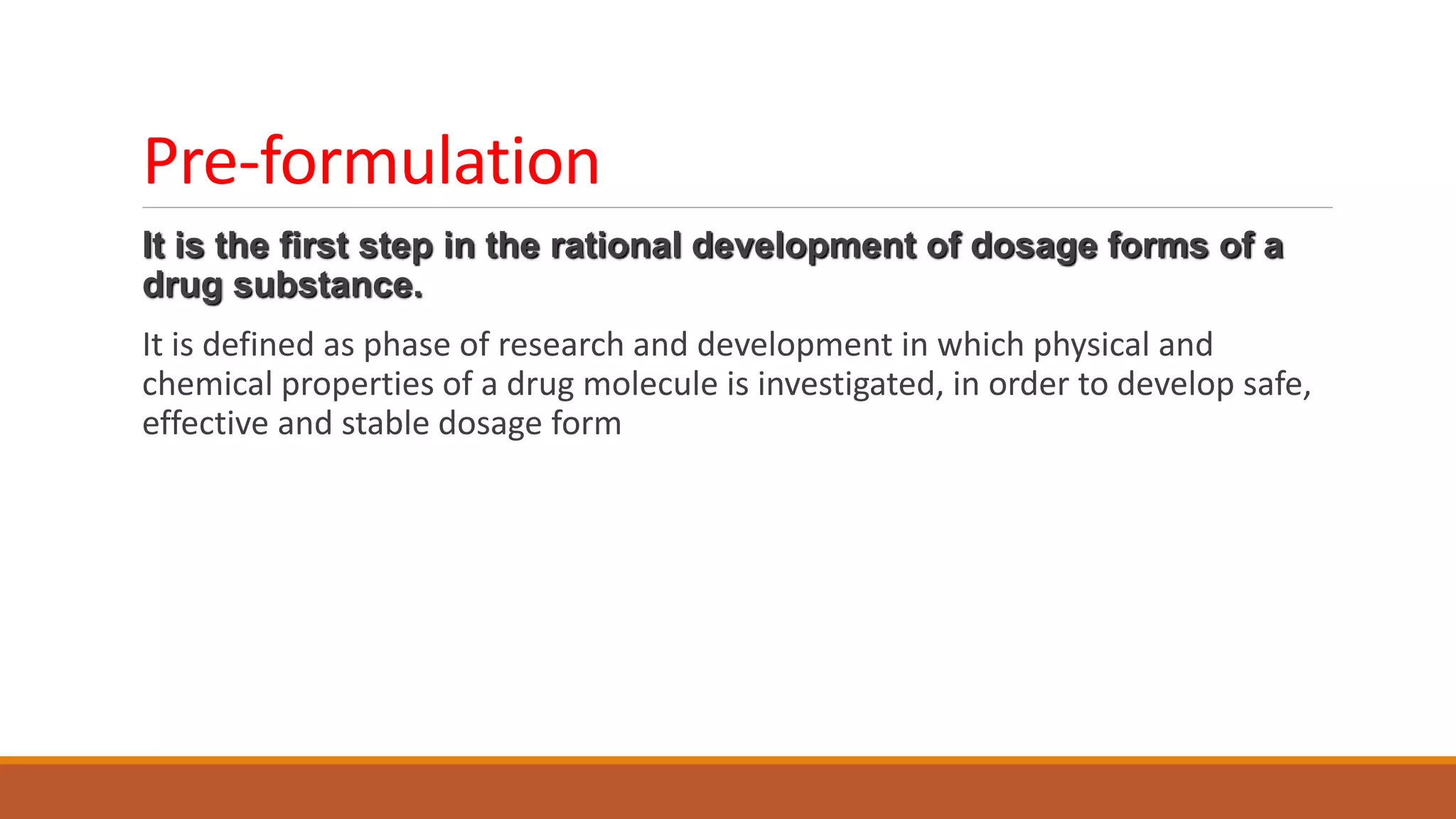
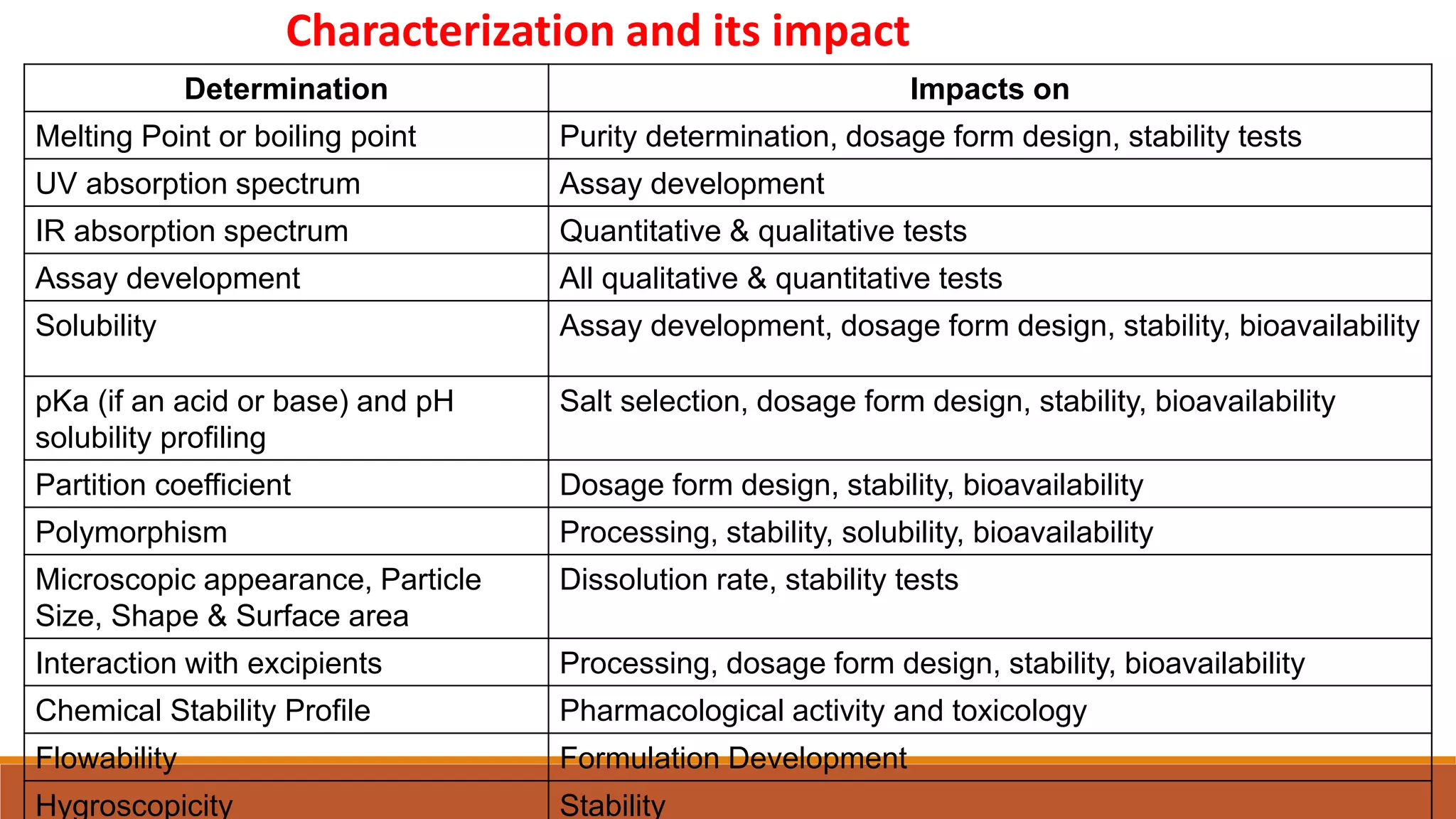
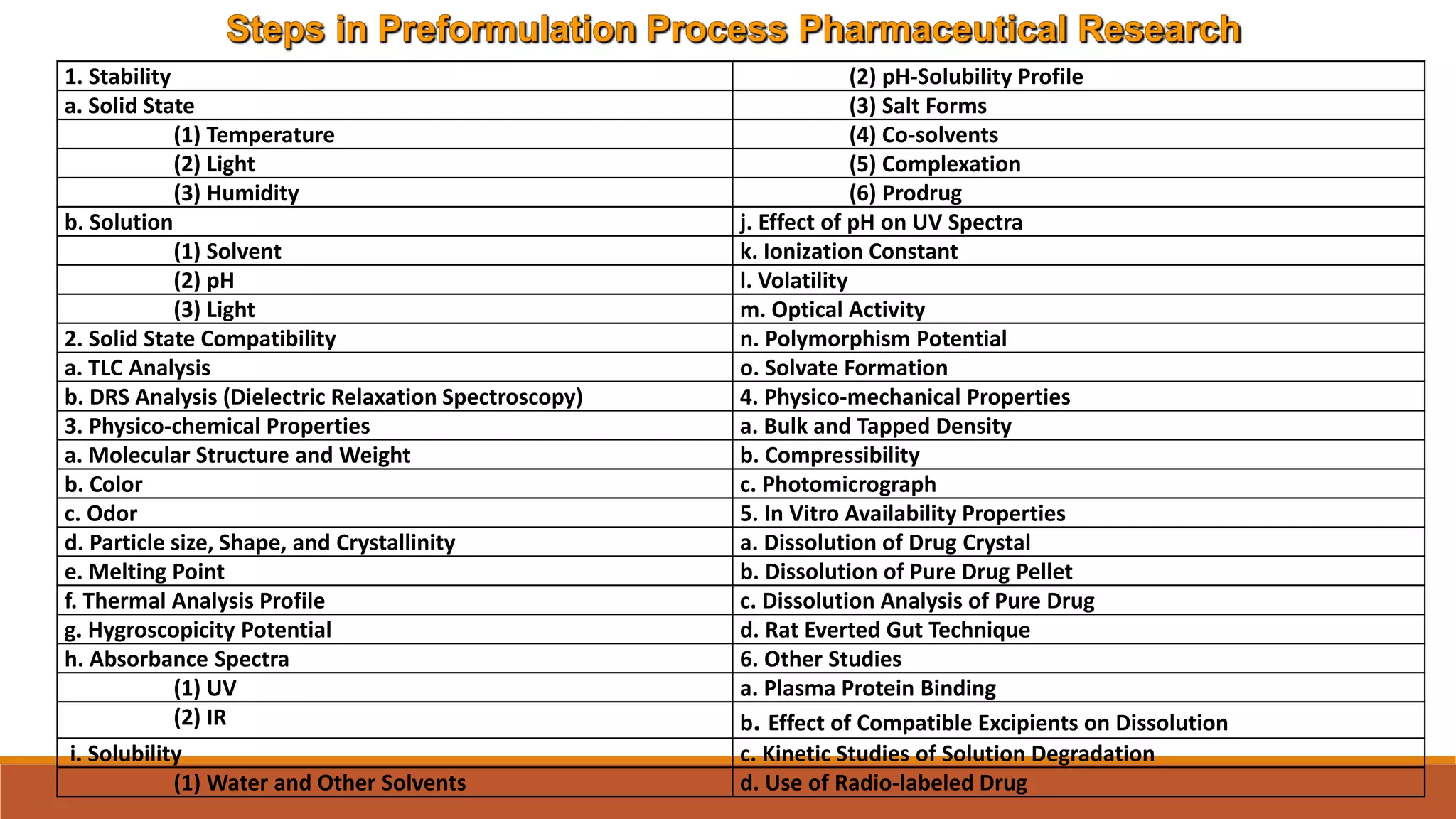
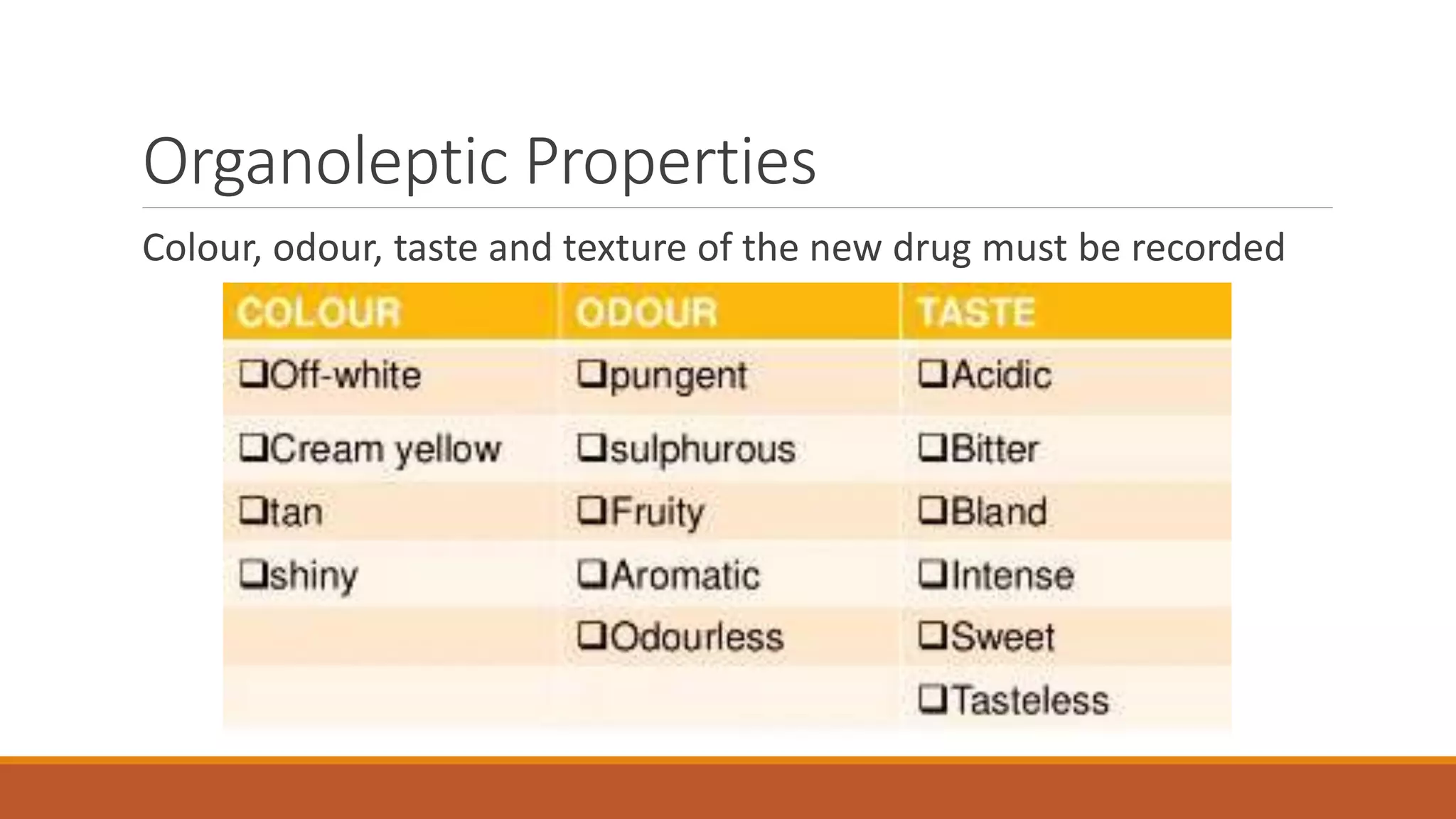
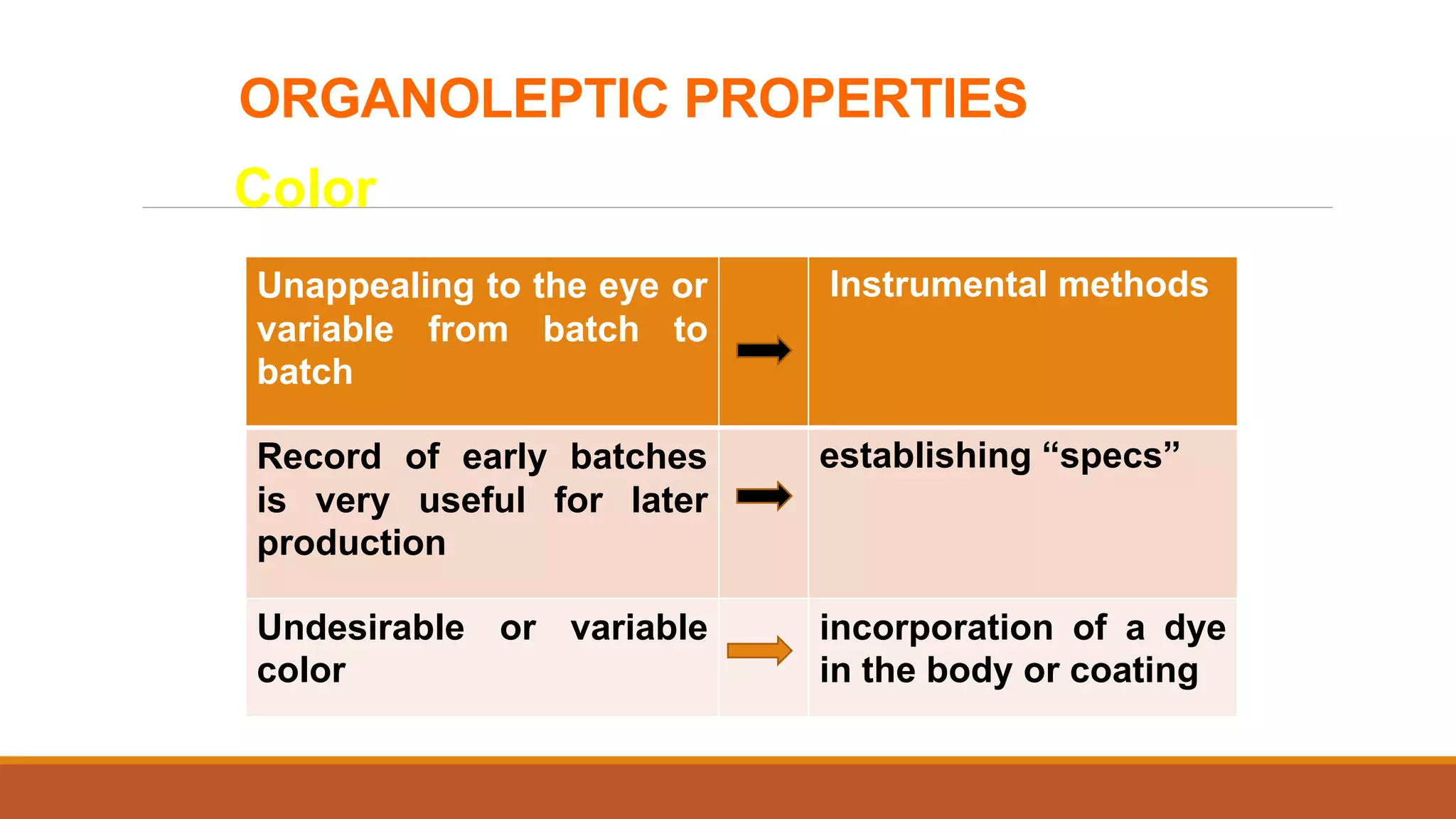
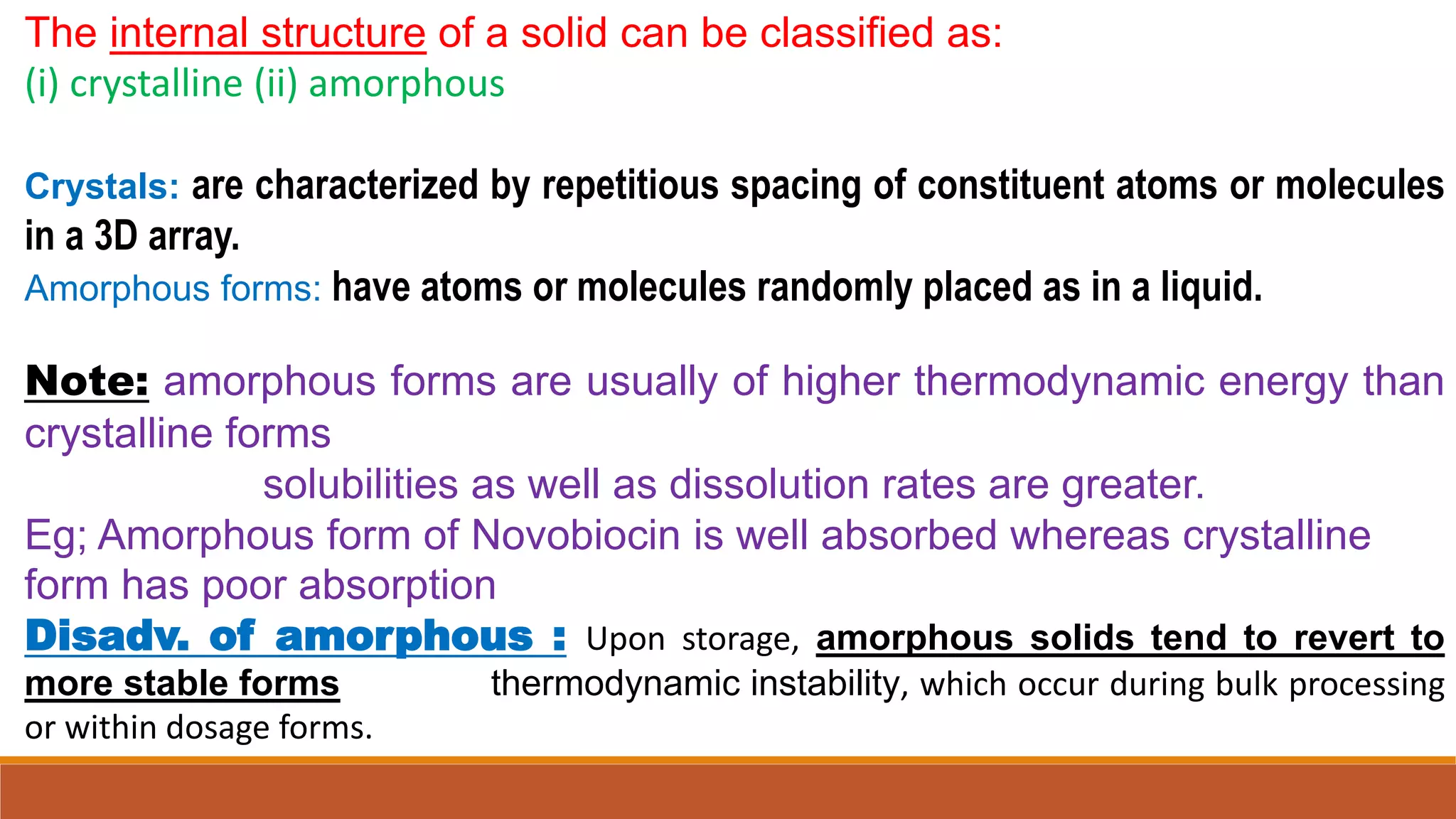
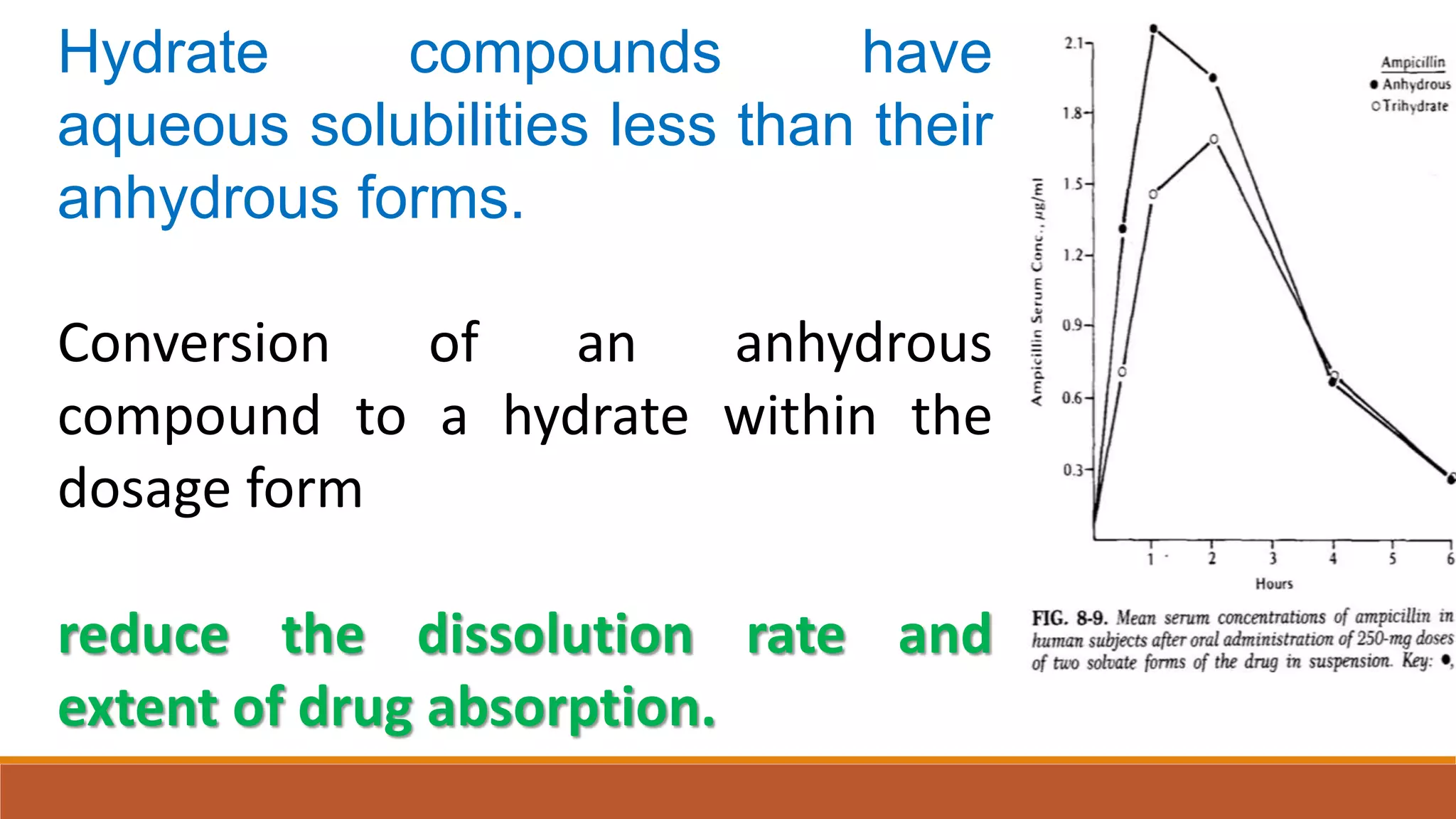
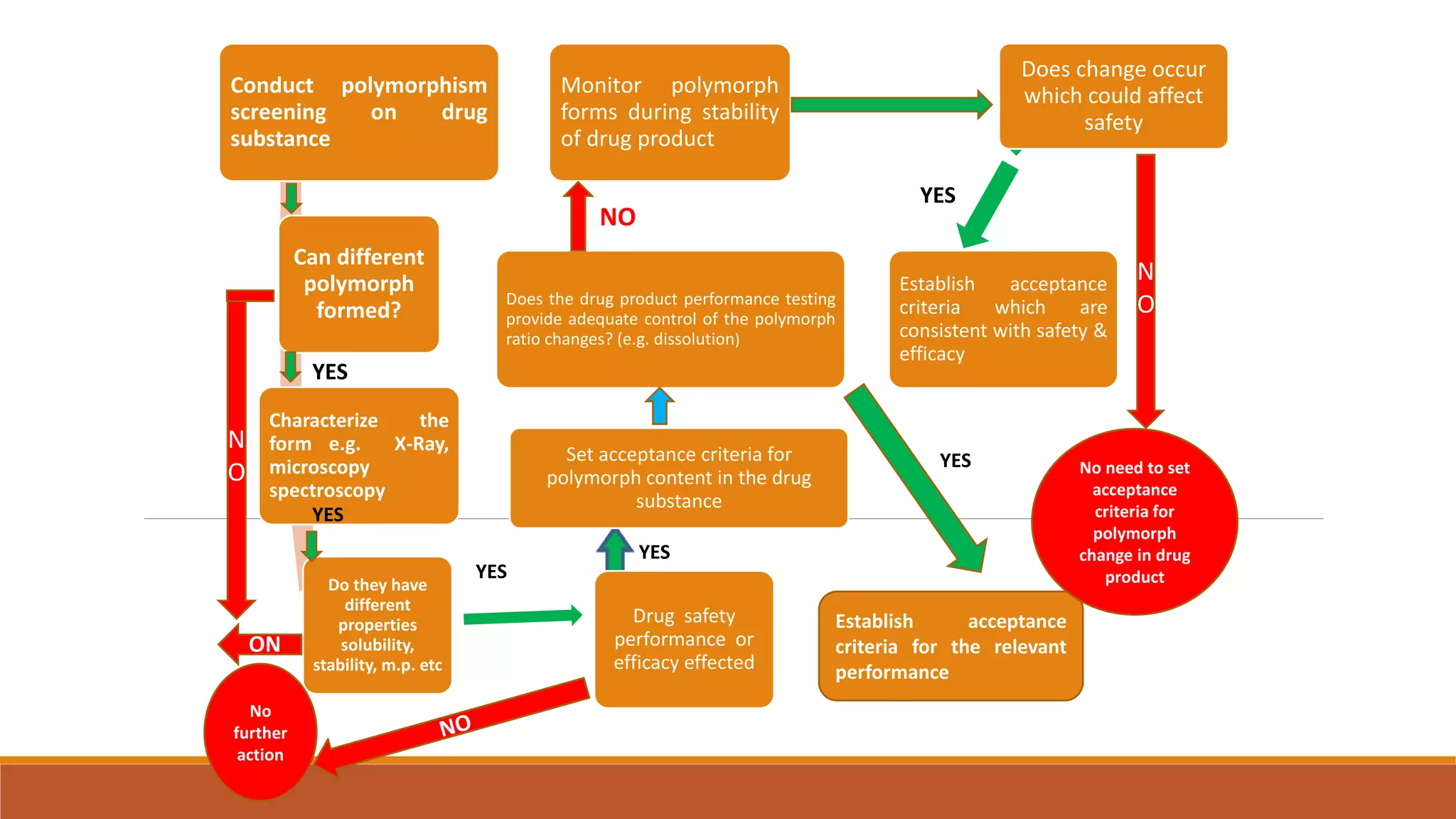
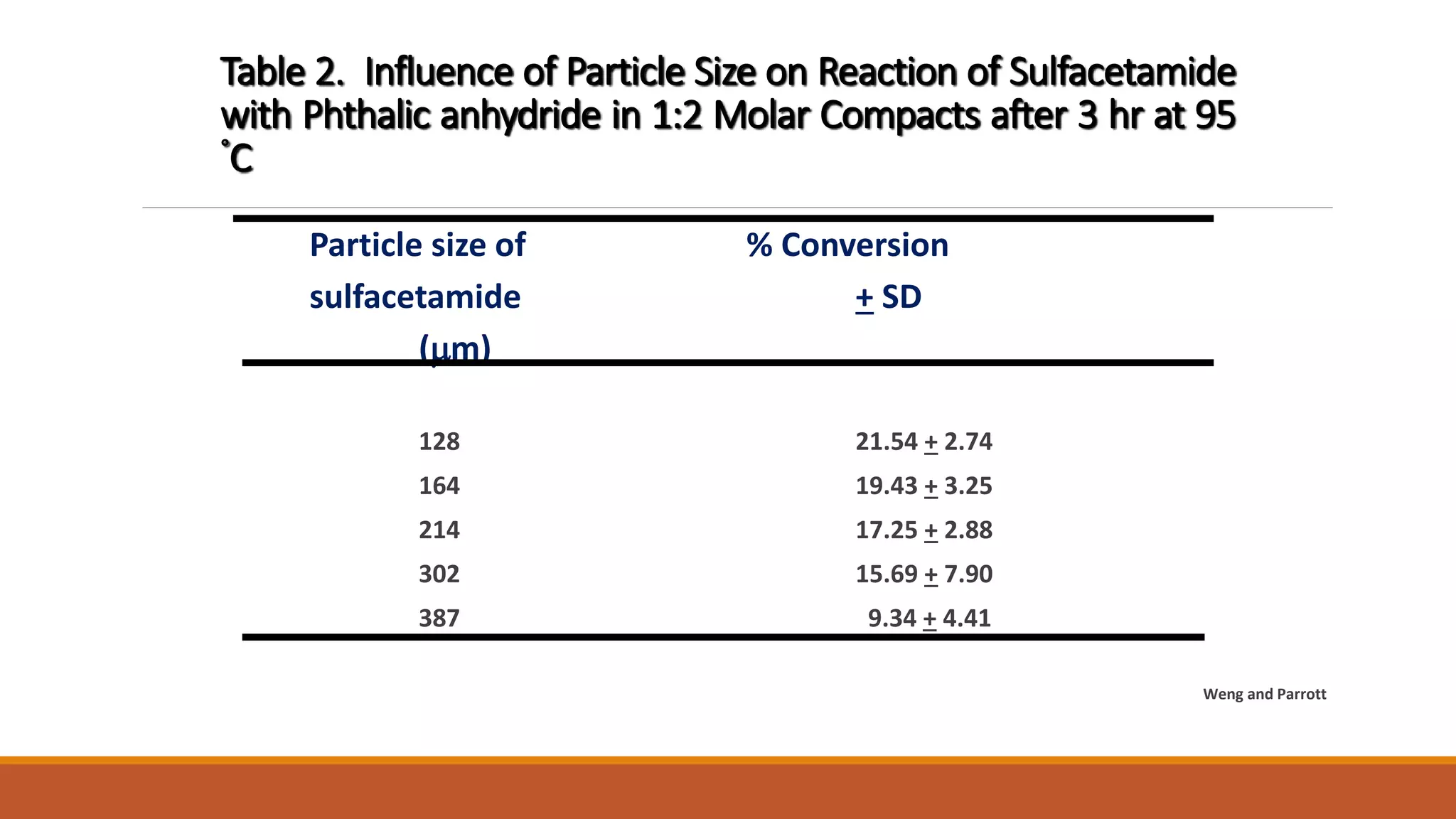
This document discusses the need for dosage forms and pre-formulation studies. It notes that dosage forms are needed to safely and conveniently deliver accurate drug doses while protecting drugs from environmental factors. Pre-formulation studies characterize the physical and chemical properties of drug substances to aid in the development of stable and effective dosage forms. These studies determine properties like solubility, stability, and compatibility with excipients. Understanding these properties provides insights to ensure quality during processing and storage.




















![Electronic means
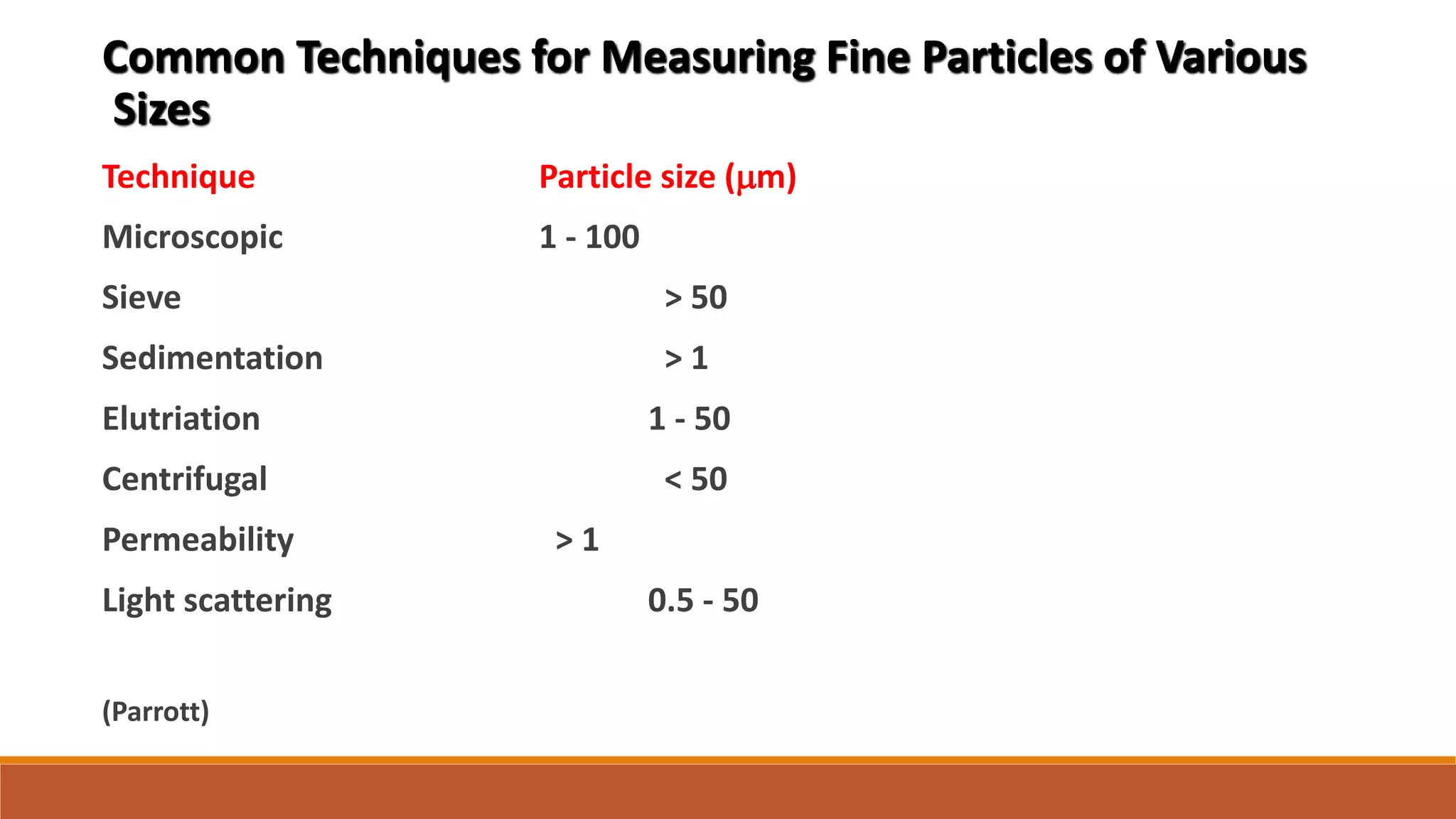
To encompass most pharmaceutical powders
ranging in size 1 - 120 mm:
Blockage of electrical conductivity path
(Coulter)
- Light blockage (HIAC) [adopted by USP]
- Light scattering (Royco)
- Laser scattering (Malvern)](https://image.slidesharecdn.com/lecture1-221010021037-7463e3dd/75/Lecture-1-pptx-36-2048.jpg)