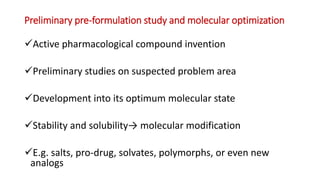
Pre-formulation studies involve evaluating the physical and chemical properties of an active pharmaceutical ingredient prior to formulation development. This helps understand the drug's behavior and identify suitable excipients and dosage forms. Key aspects of pre-formulation studies include evaluating solubility, stability, dissolution rate, and compatibility with excipients. Techniques like DSC, FTIR, XRD are used to analyze properties like crystallinity, polymorphism and for compatibility testing. Together, pre-formulation studies provide critical data to inform the rational development of a stable and effective dosage form.






![Solubility in various pH
Every new drug must be determined as a function of pH ,why?
• Over the physiological pH range of 1-8.
• For relatively insoluble drugs , rate of dissolution depends on its
solubility which can influence its biological absorption.
• Stability during processing
Henderson -Hassel Bach equation to find ratio of ionised/unionised drug
• pH = pKa + log [(un-ionized drug) / (ionized drug)]→basic drug
• pH = pKa + log [(ionized drug) / (un-ionized drug)]→acidic drug](https://image.slidesharecdn.com/01-preformulationstudies2-210304053558/85/Preformulation-studies-8-320.jpg)
















![Partition coefficient
Partition Coefficient(P) = [Organic] / [Aqueous]
• Log P= log10 (Partition Coefficient)
Log P = 1 → 10:1 Organic: Aqueous
Log P = 0 → 1:1 Organic: Aqueous
Log P = -1 → 1:10 Organic: Aqueous](https://image.slidesharecdn.com/01-preformulationstudies2-210304053558/85/Preformulation-studies-25-320.jpg)

![The relative concentration of the ionized and the unionized form
Henderson Hassel Bach equation
pH = pKa + log [unionized form] / [Ionized form] FOR BASES
pH = pKa + log [ionized form]/ [unionized form] FOR ACIDS
Ratio can be determined
• Potentiometric pH-Titration,
• pH-Spectrophotometry Method,
• pH-Solubility Analysis.](https://image.slidesharecdn.com/01-preformulationstudies2-210304053558/85/Preformulation-studies-27-320.jpg)

















