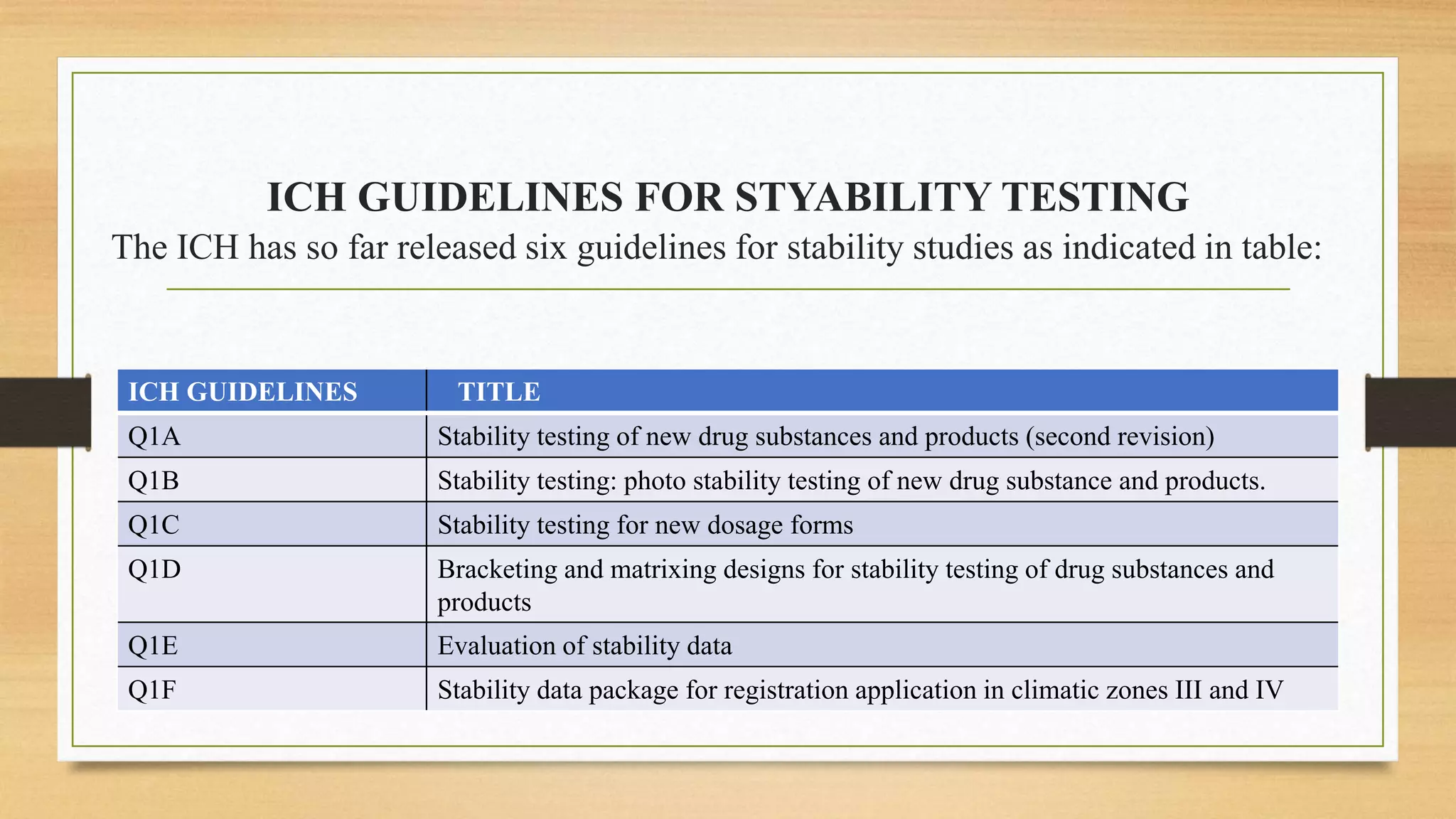
The document discusses stability studies in pharmaceuticals, focusing on the effects of environmental conditions on drug quality and shelf life. It outlines the primary factors affecting stability, types of stability (chemical, physical, microbiological, therapeutic, and toxicologic), and regulatory requirements for stability testing. Additionally, it covers long-term and accelerated stability studies, including guidelines from ICH and WHO, and the significance of degradation pathways in pharmaceutical formulations.