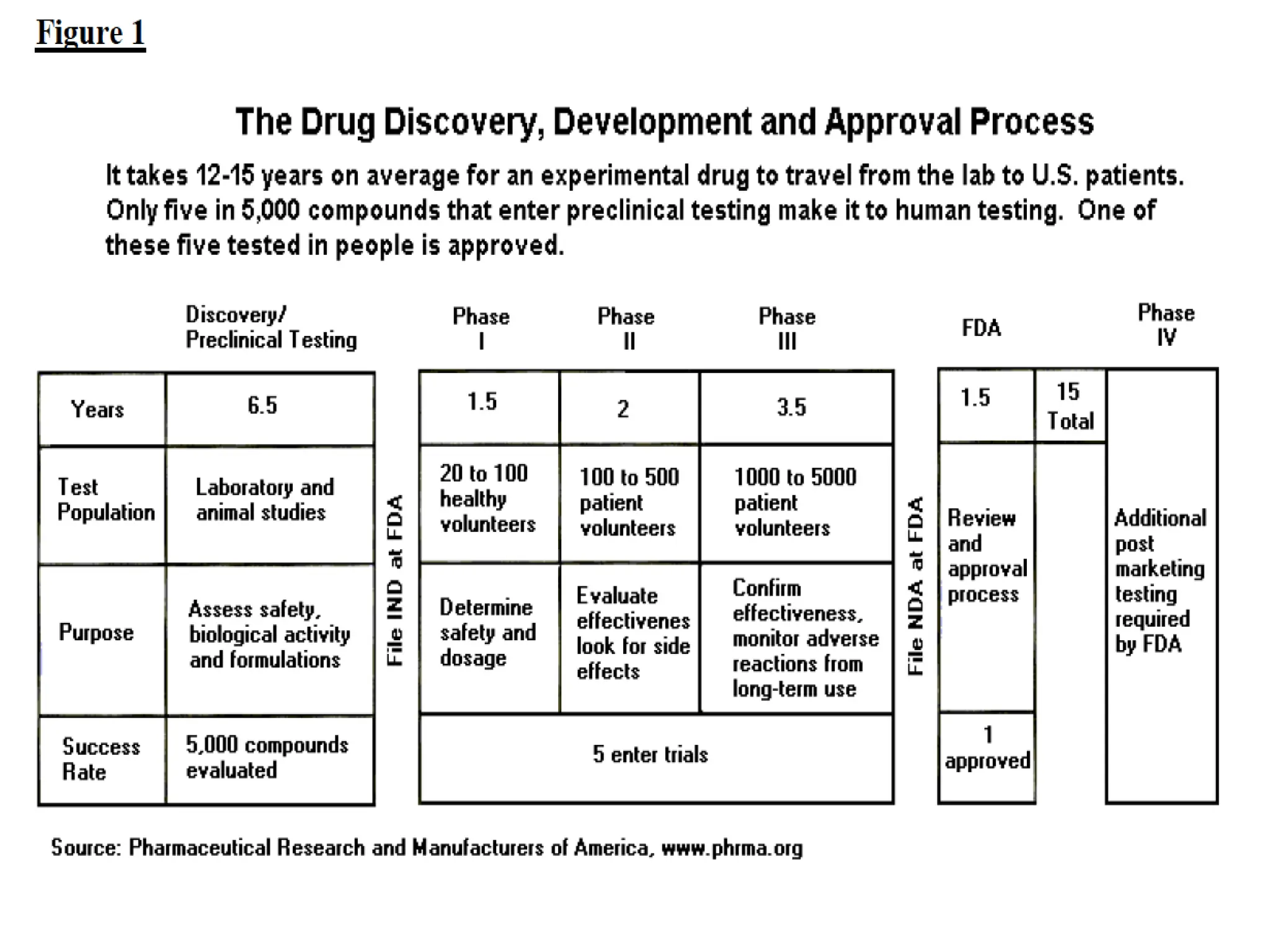
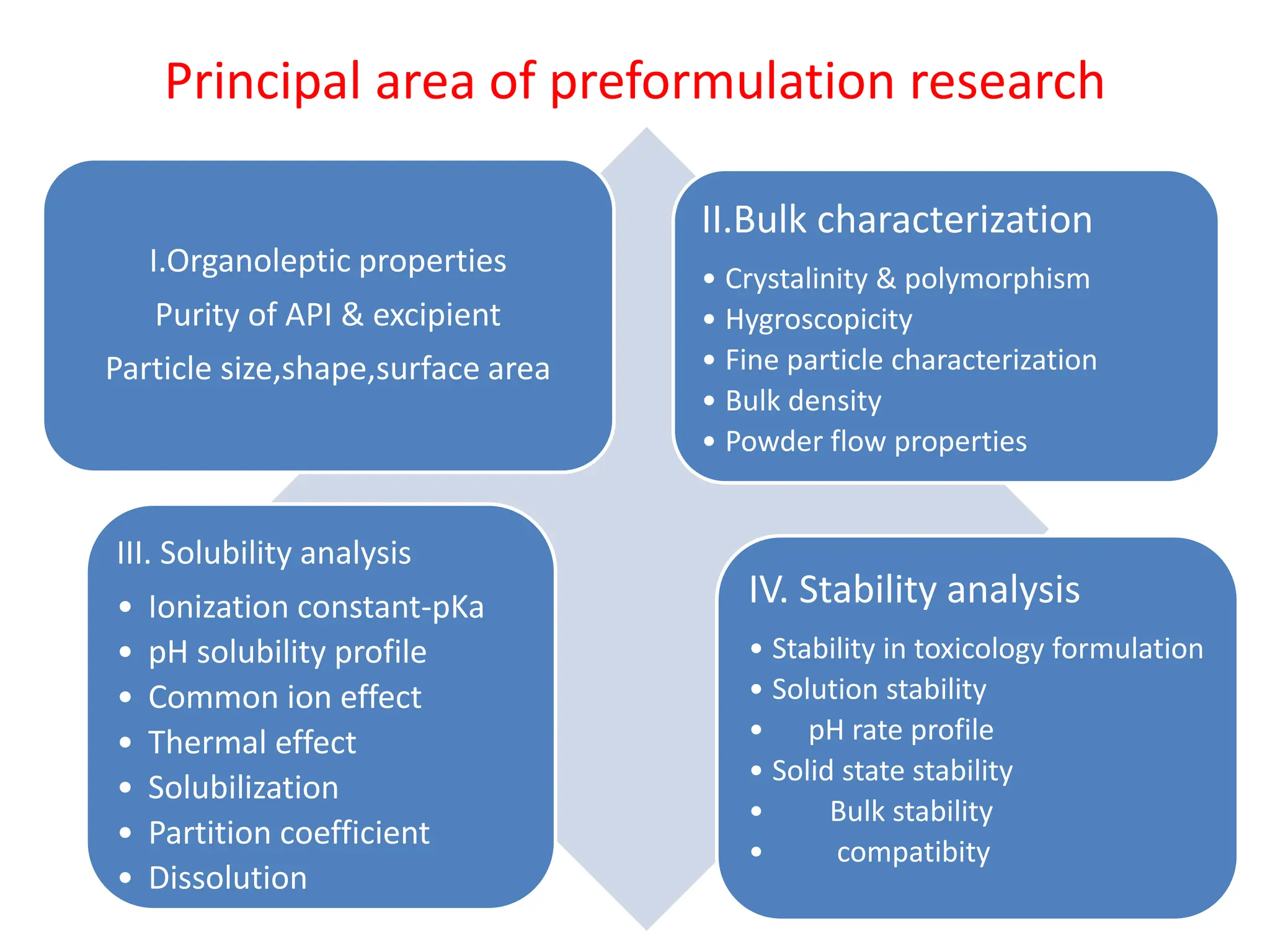
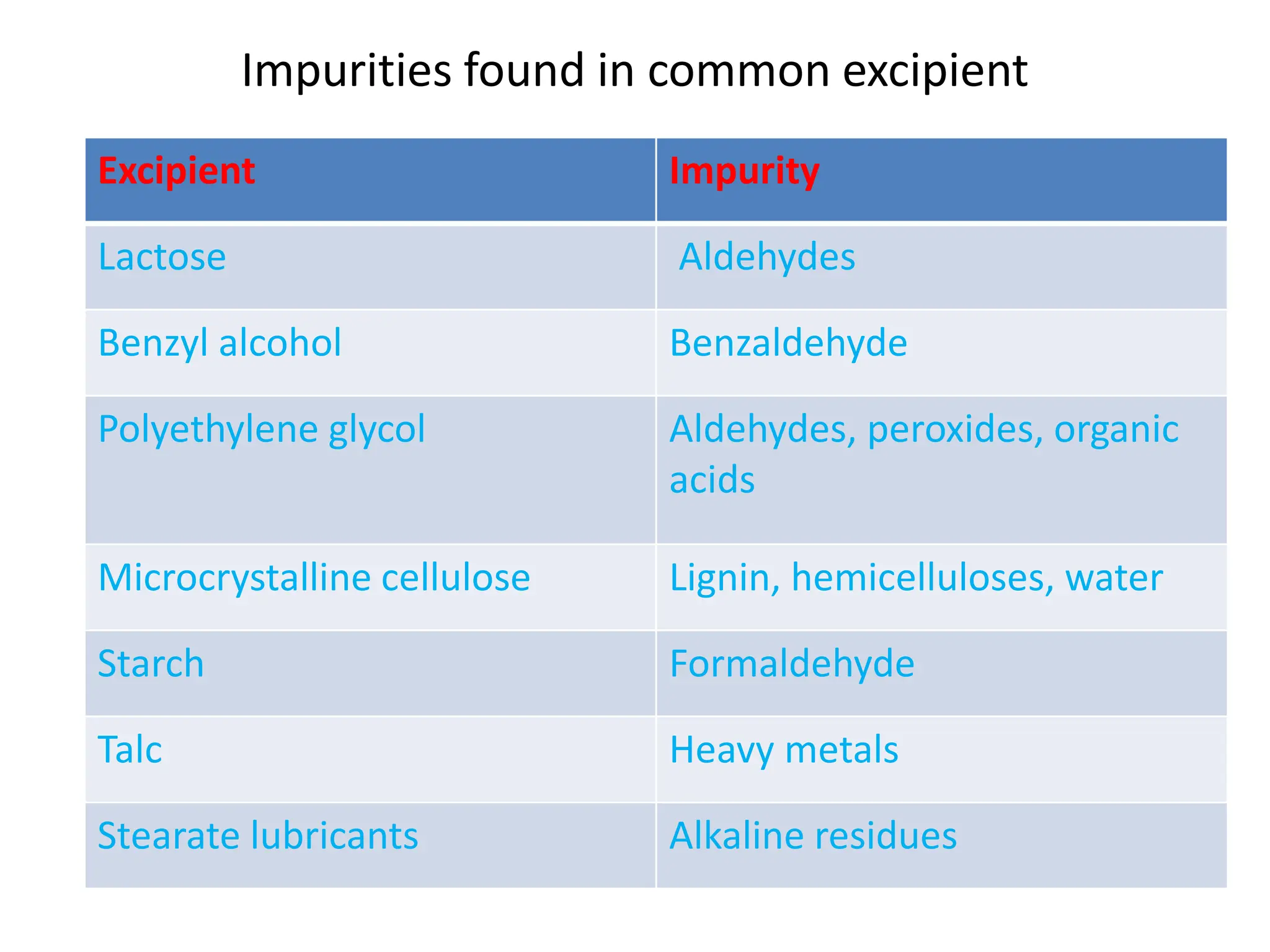
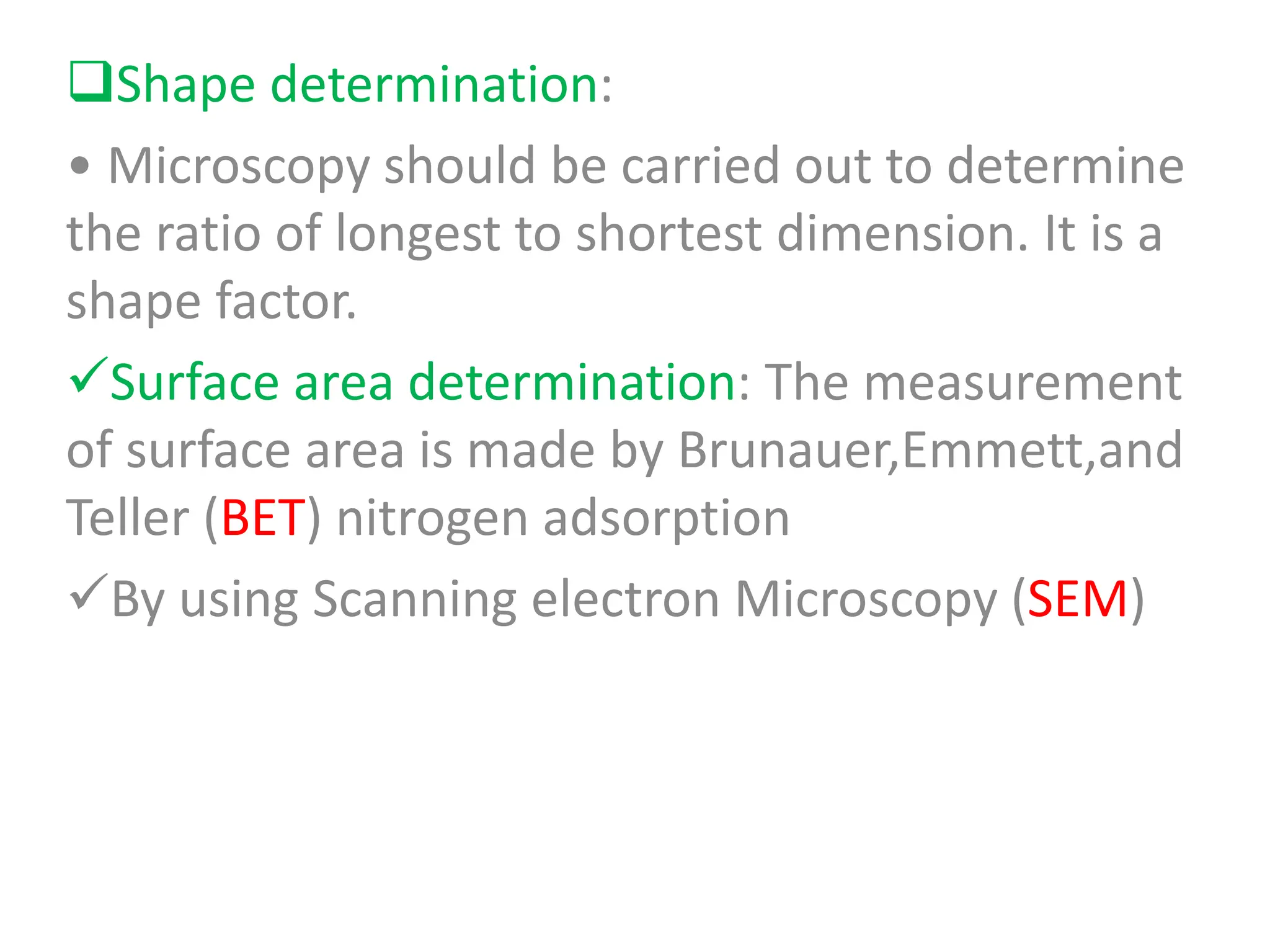
The document discusses preformulation, which involves characterizing a drug's physicochemical properties to aid in developing a stable and effective dosage form. Some key goals of preformulation testing are to determine solubility, stability, and compatibility with excipients. Various analytical techniques are used to evaluate properties like polymorphism, particle size, and purity that can impact drug performance. The results of preformulation studies provide critical information to formulation scientists in designing an optimal drug delivery system.