1) Solubility is the maximum amount of a substance that dissolves in a solvent to form a saturated solution at a given temperature and pressure.
2) Solubility is ideally measured at 4°C and 37°C to ensure physical stability and support biopharmaceutical evaluation. Solubility below 1 mg/ml indicates poor absorption and need for preformulation studies.
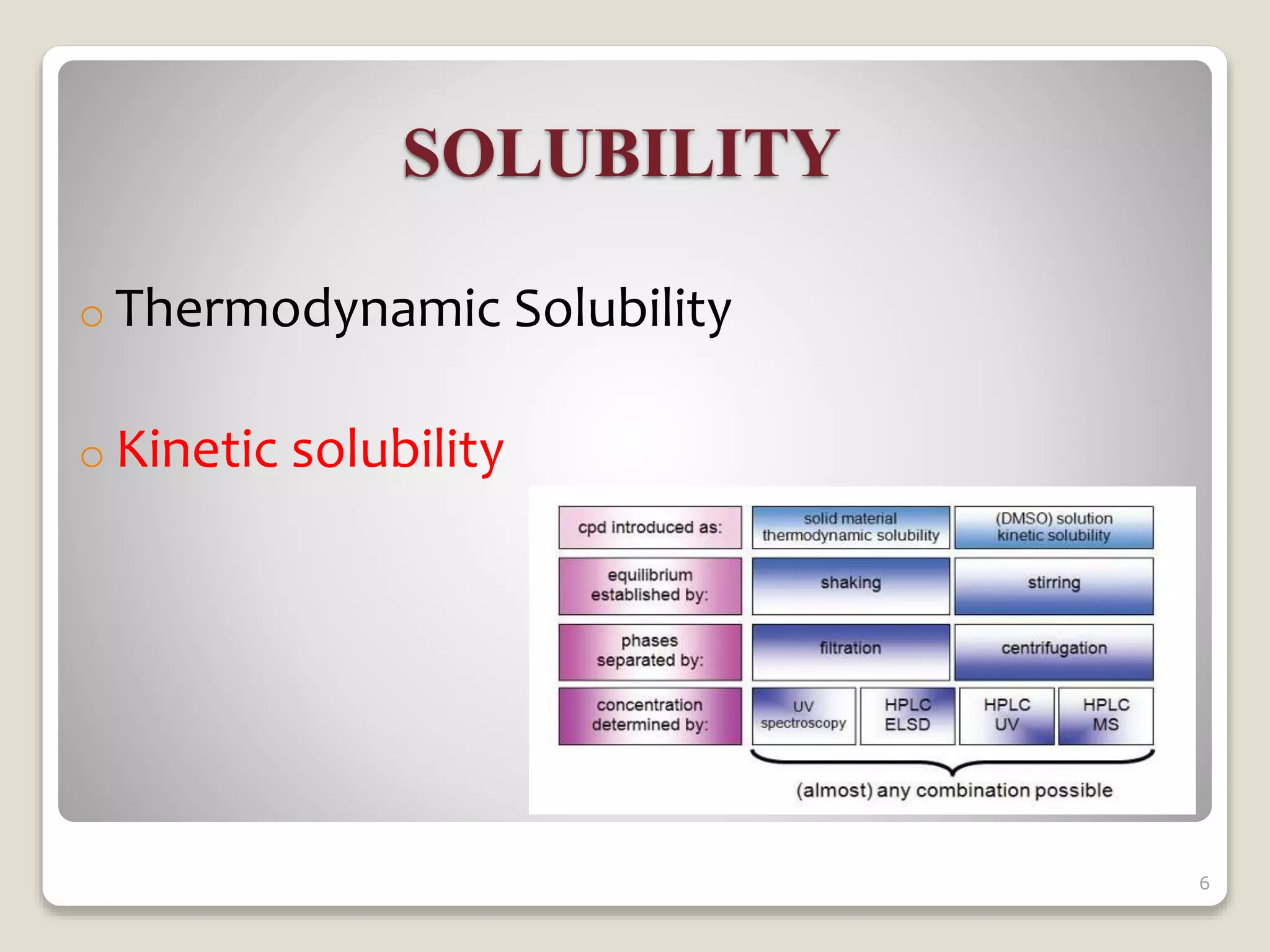
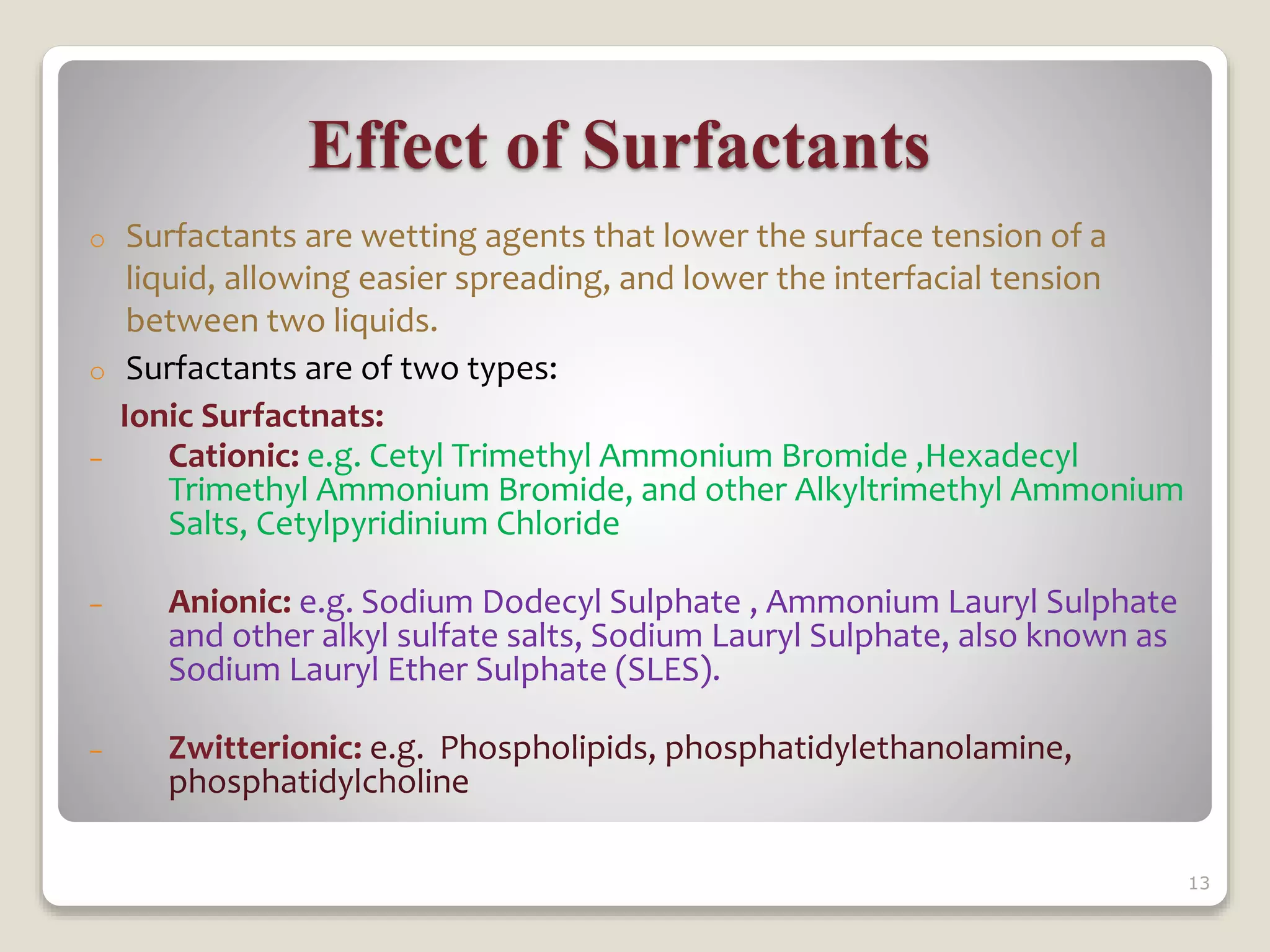
3) Preformulation solubility studies focus on the drug solvent system and include determining properties like intrinsic solubility, pH solubility profiles, effects of surfactants, and temperature dependence to understand a drug's solubility and dissolution behavior.














![pKa Determination
Can be calculated by Henderson Hasselbalch equation-
18
For basic drugs….pH= pKa+ log [unionized drug]
[ionized drug}
For acidic drugs….pH= pKa+ log [ionized drug]
[unionized drug}](https://image.slidesharecdn.com/solubilityanditsdetermination-160904111326/75/Solubility-and-its-determination-18-2048.jpg)