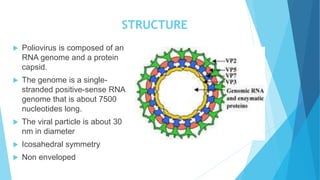
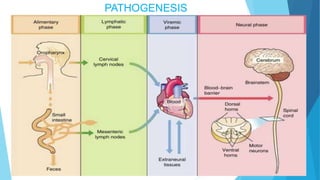
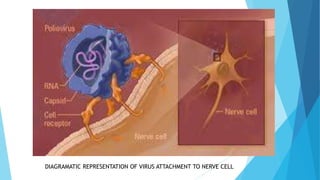
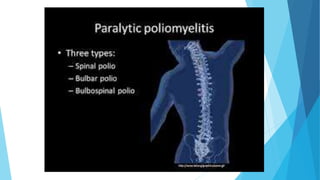
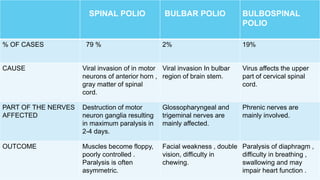
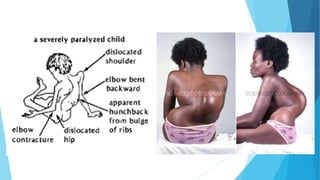
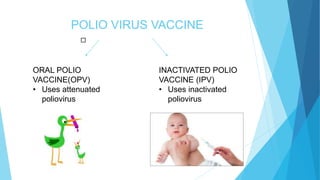
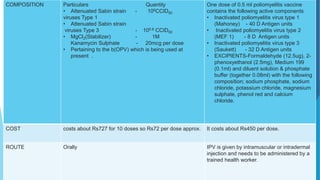
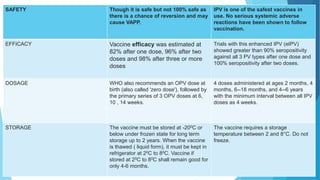
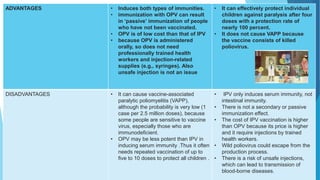
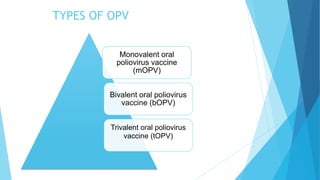
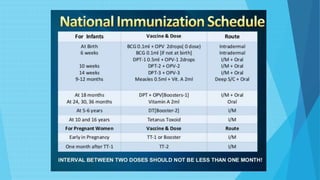
The document discusses the poliovirus, which causes poliomyelitis (polio). It causes paralysis in about 1% of cases. Two main vaccines were developed - the live attenuated oral polio vaccine (OPV) by Albert Sabin in the 1950s and the inactivated polio vaccine (IPV) by Jonas Salk in the 1950s. OPV uses weakened live virus and provides both intestinal and serum immunity but carries a small risk of vaccine-derived polio. IPV uses killed virus and provides only serum immunity but is very safe. Both vaccines have been highly effective at reducing polio worldwide.