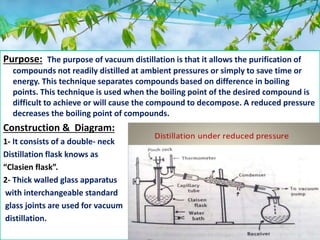
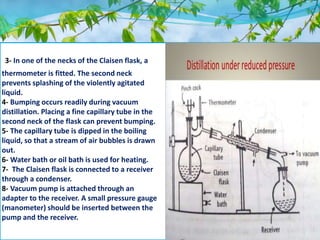
Vacuum distillation allows purification of compounds that cannot be readily distilled under normal atmospheric pressure or that may decompose at high temperatures. It works by reducing pressure above the boiling liquid, lowering the boiling point so compounds evaporate and separate based on differences in their boiling points. Key advantages are faster processing, ability to distill higher boiling point solvents without damage, and improved separation, yield, and purity. It finds applications in separating thermolabile substances and preparing extracts while preserving active constituents.