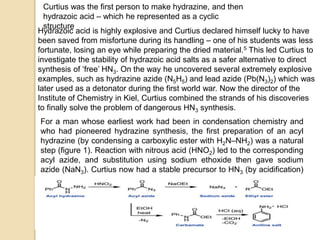
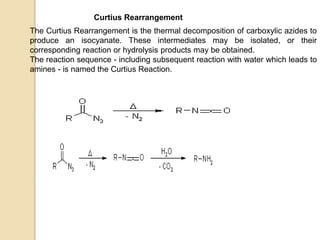
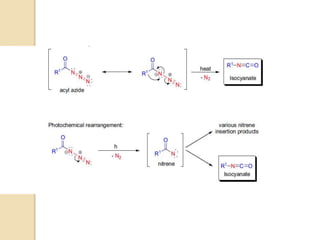
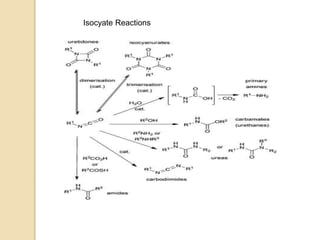
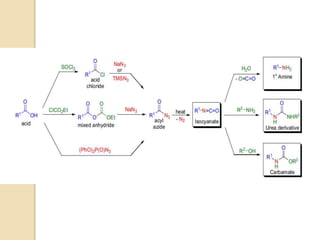
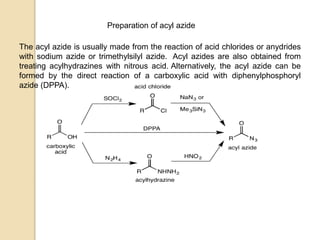
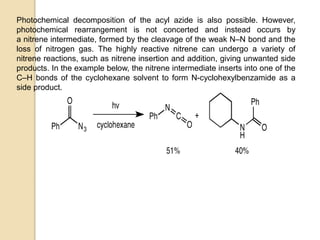
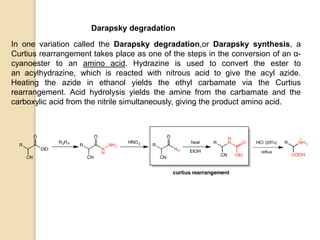
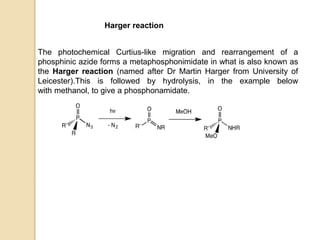
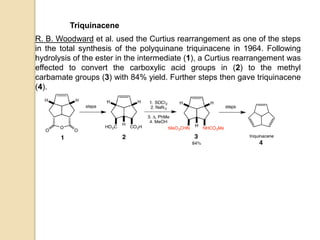
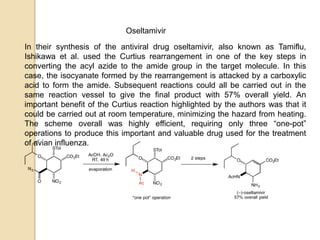
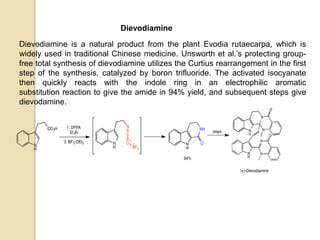
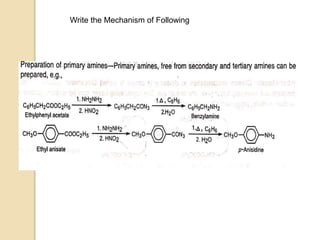
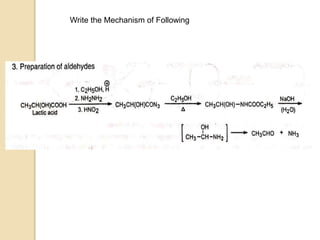
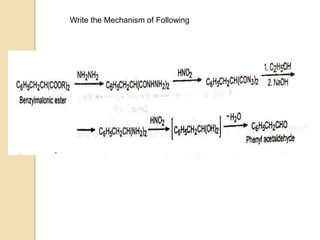
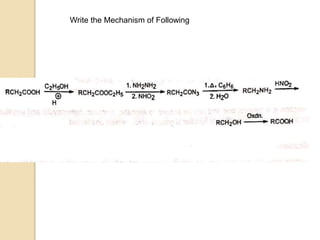
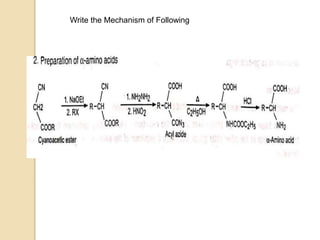
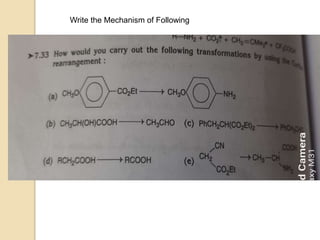
The Curtius rearrangement is a reaction where a carboxylic acid is converted to an isocyanate through an acyl azide intermediate. The isocyanate then undergoes further reactions to form other products. Theodor Curtius discovered this reaction in the late 19th century. It has since been used in the synthesis of important compounds like the antiviral drug oseltamivir and the natural product dievodiamine. The reaction can proceed through thermal or photochemical decomposition of the acyl azide.