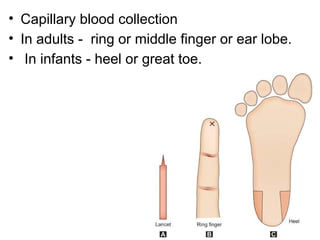
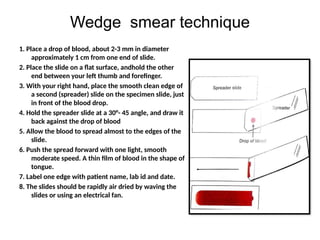
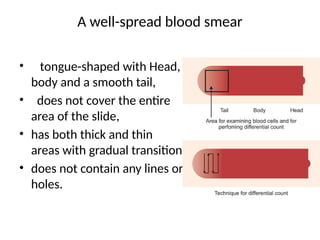
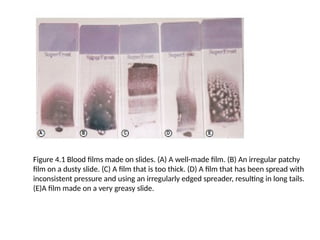
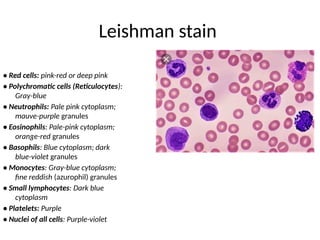
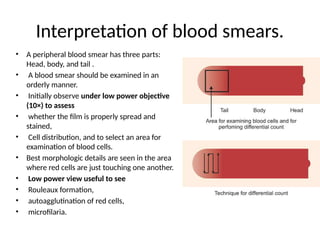
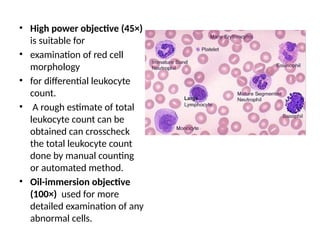
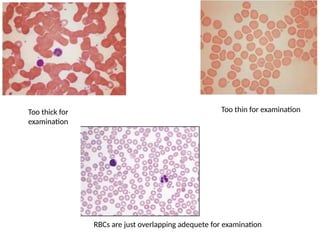
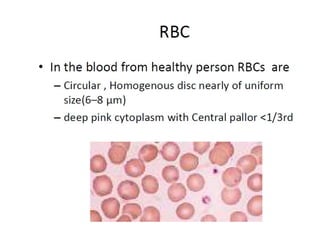
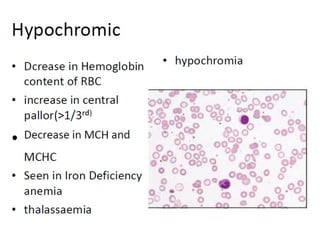
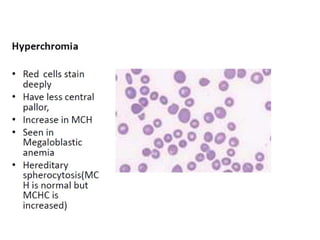
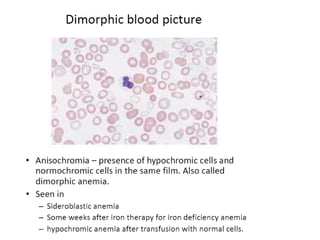
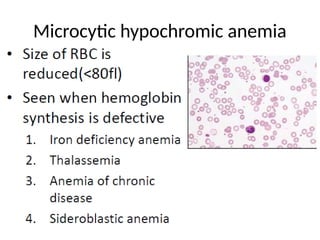
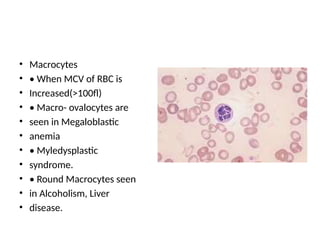
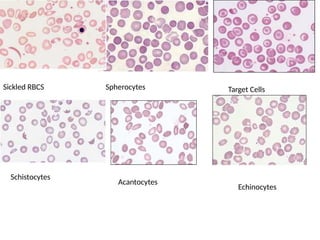
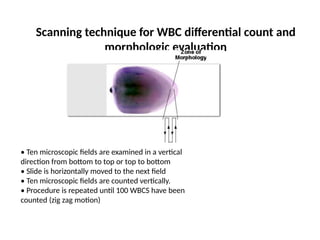
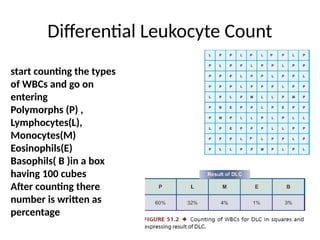
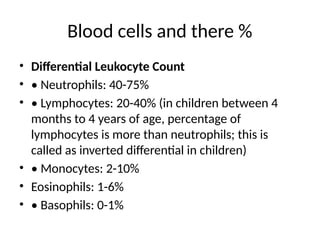
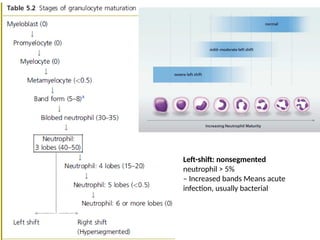
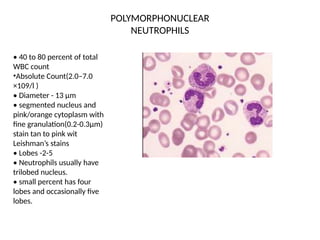
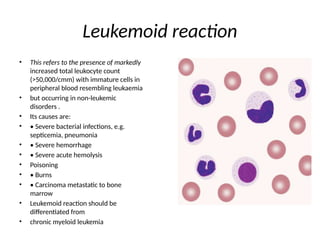
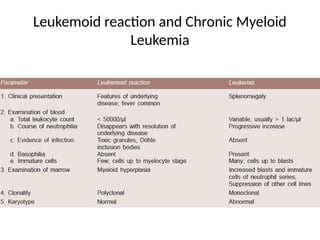
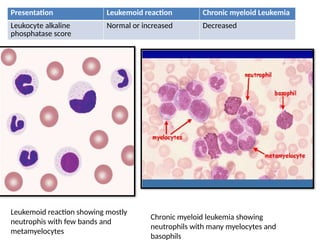
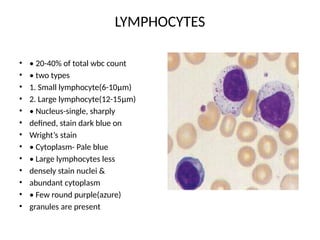
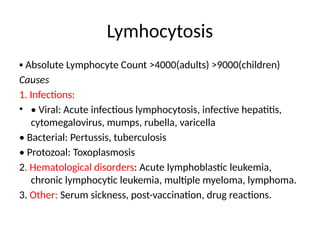
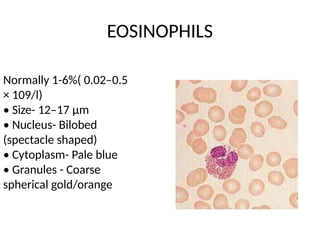
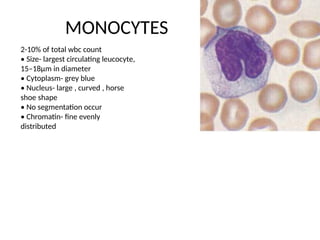
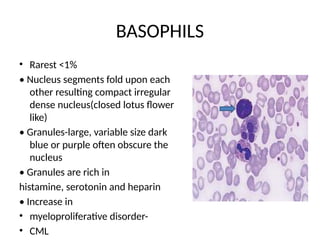
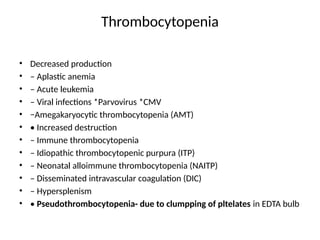
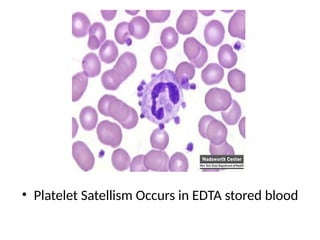
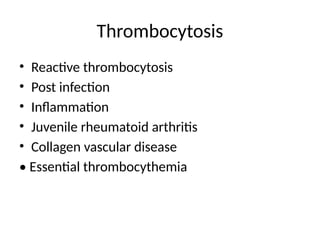
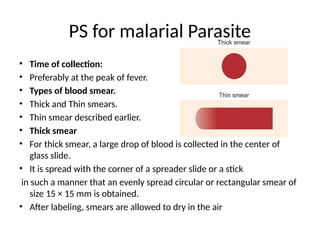
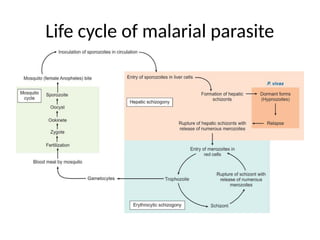
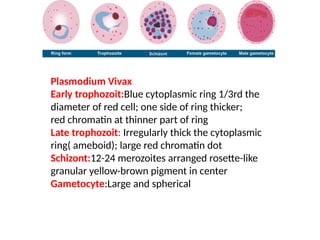
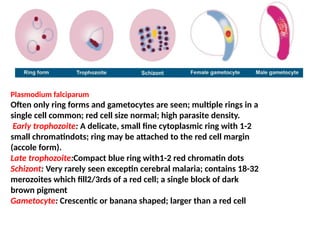
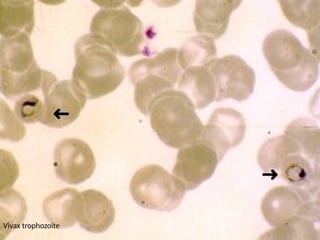
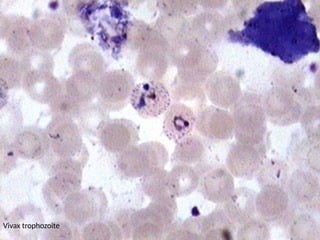
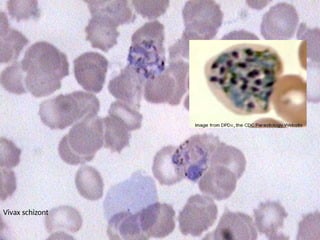
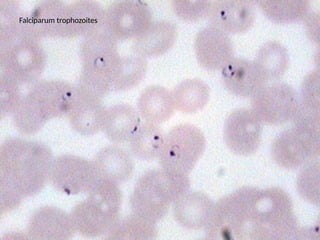
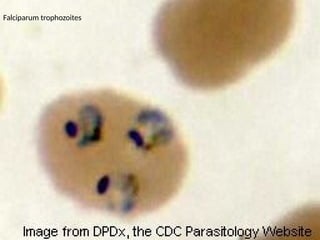
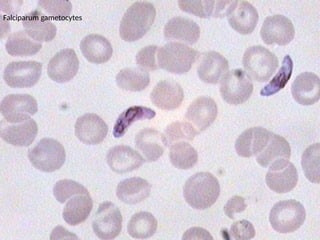
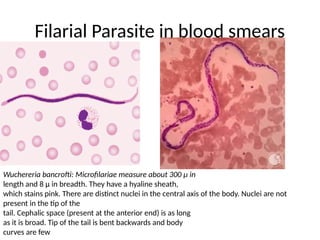
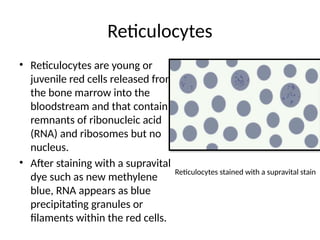
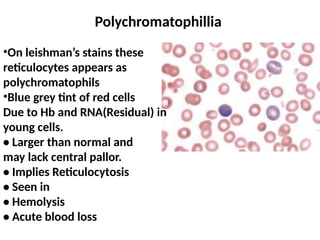
The document outlines basic haematological techniques focusing on peripheral blood smear examination, including specimen collection, slide preparation, staining, and interpretation of blood smears. It details the importance of identifying various conditions through blood films, such as leukocytosis and anemia, alongside the specific microscopic features of blood cells. Techniques for performing and analyzing the smear, as well as the relevant staining methods and interpretations of the results, are thoroughly covered.