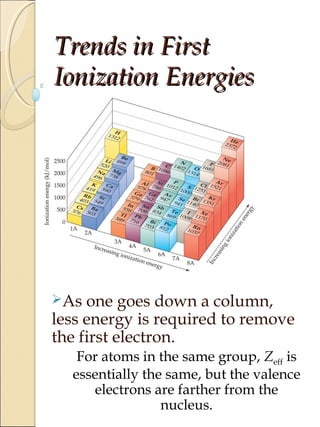
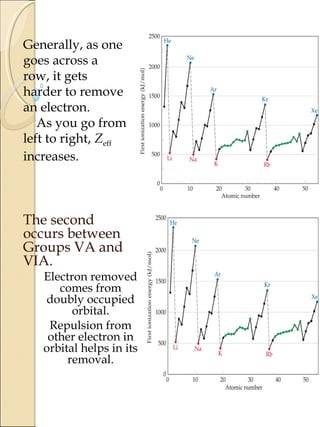
1) The document discusses trends in ionization potential and electron affinity across the periodic table. Ionization potential generally increases from left to right in a period as nuclear charge increases and atomic radius decreases. It also decreases down a group as atomic size increases.
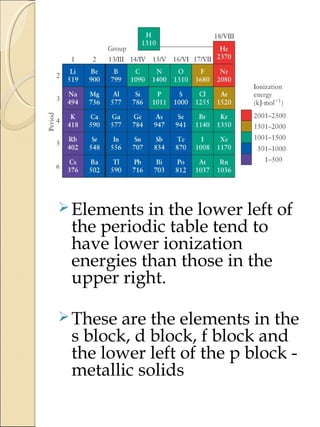
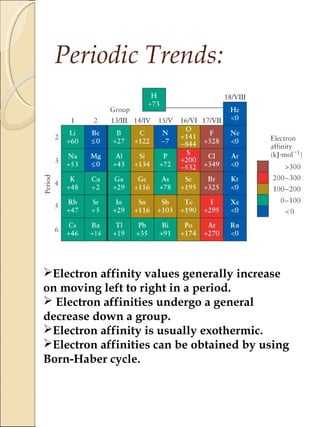
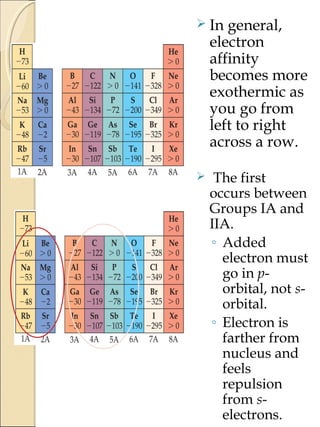
2) Electron affinity generally increases from left to right as nuclear charge increases but decreases down a group as atomic size increases. Elements in the lower left of the periodic table tend to have lower ionization potentials and are more metallic.
3) Factors that influence ionization potential and electron affinity include effective nuclear charge, atomic size, shielding effects, and stability of electron configurations.