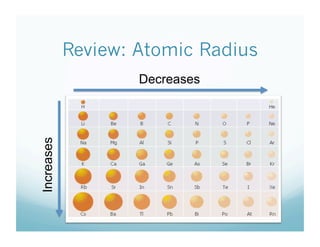
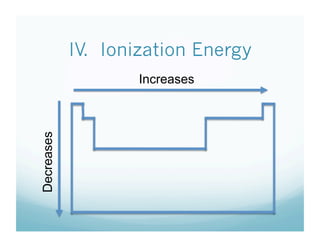
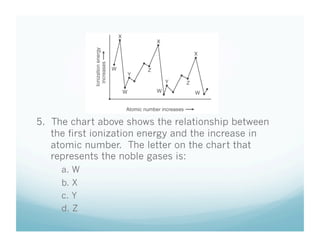
The document provides information about ionization energy and its trends across the periodic table. It defines ionization energy as the energy required to remove an electron from an atom. It states that ionization energy increases across a period as atomic radius decreases, and decreases down a group as atomic radius increases. It also notes that second and third ionization energies are always higher than first ionization energy due to the increased pull on remaining electrons.