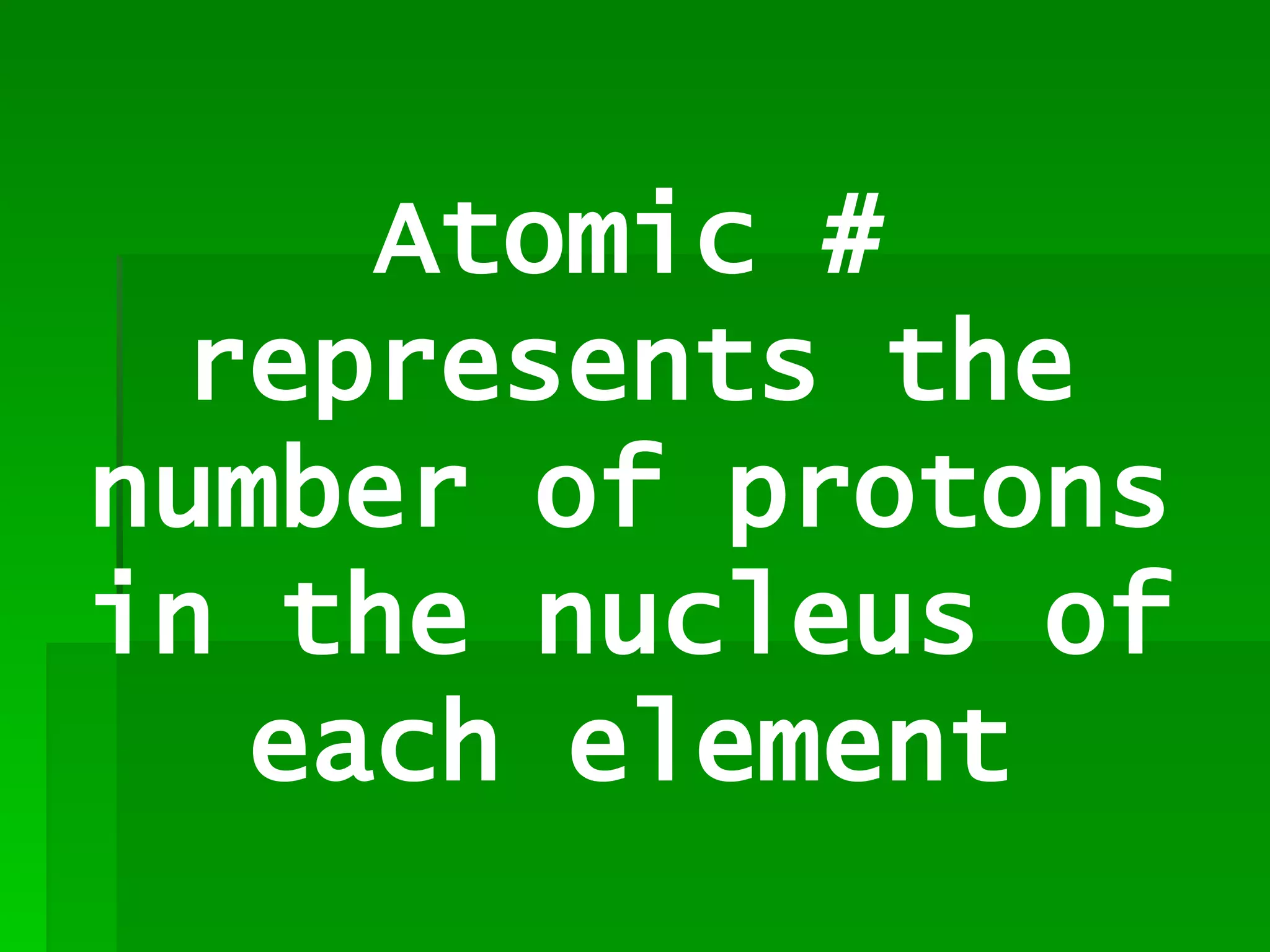
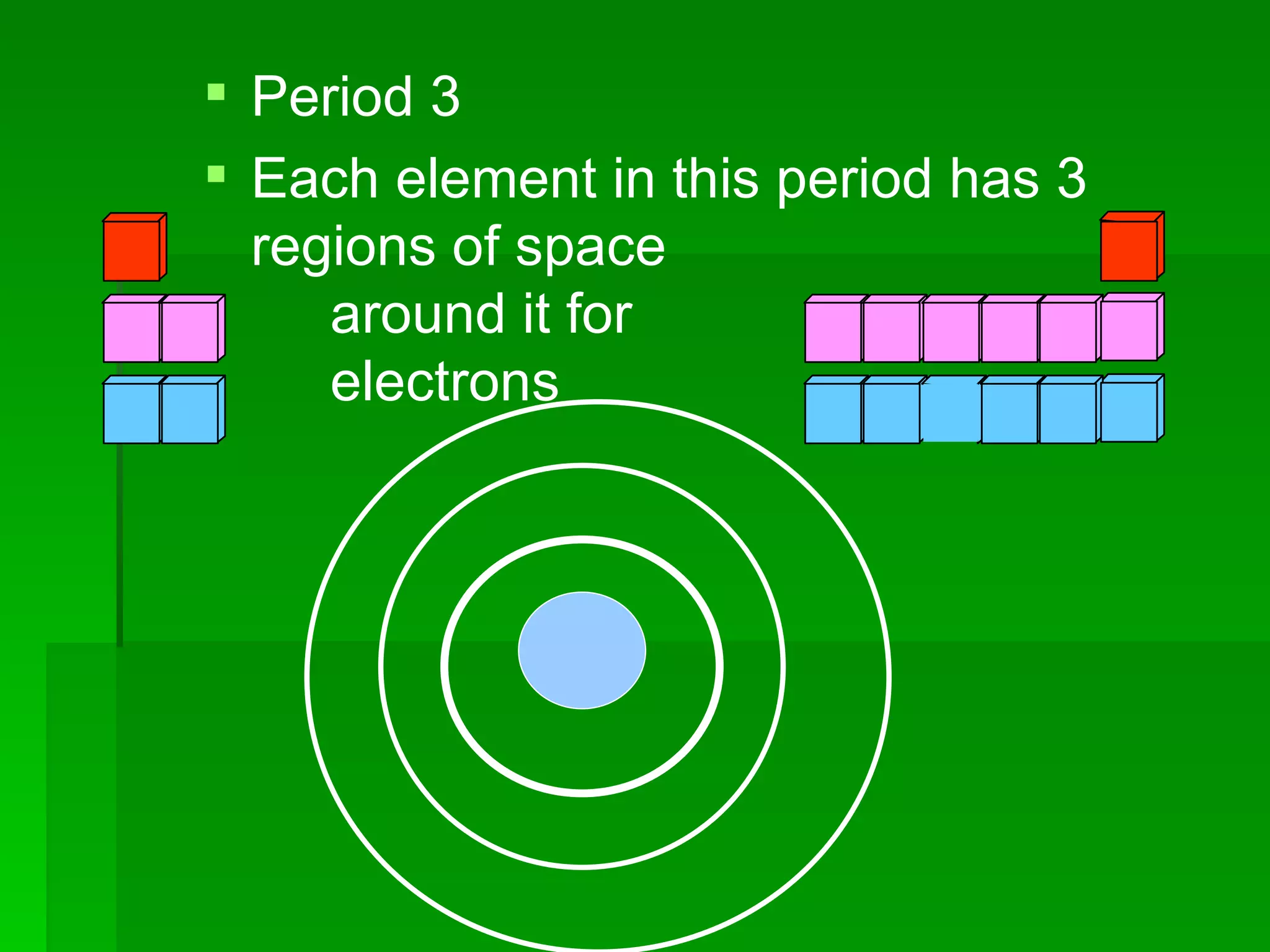
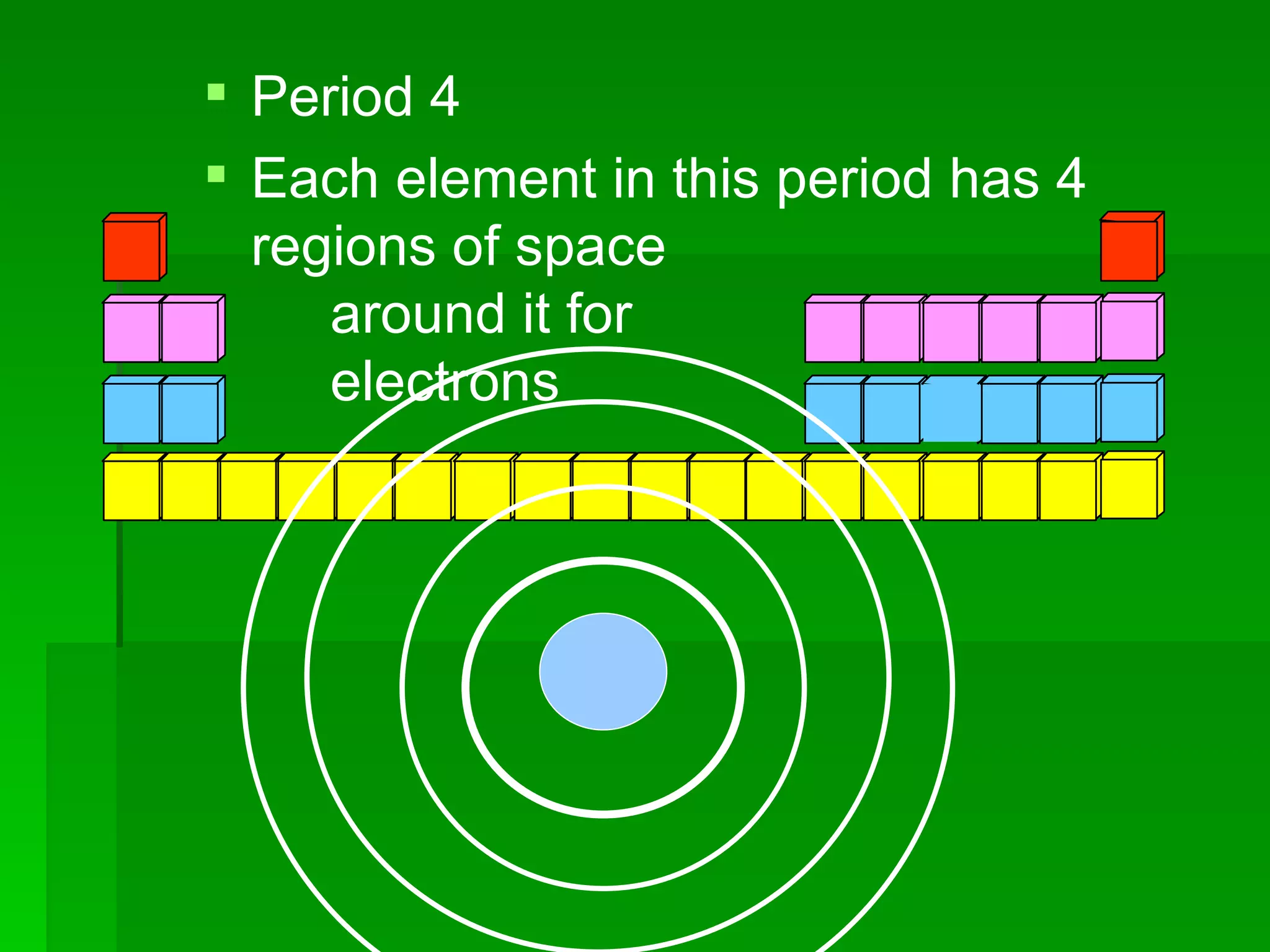
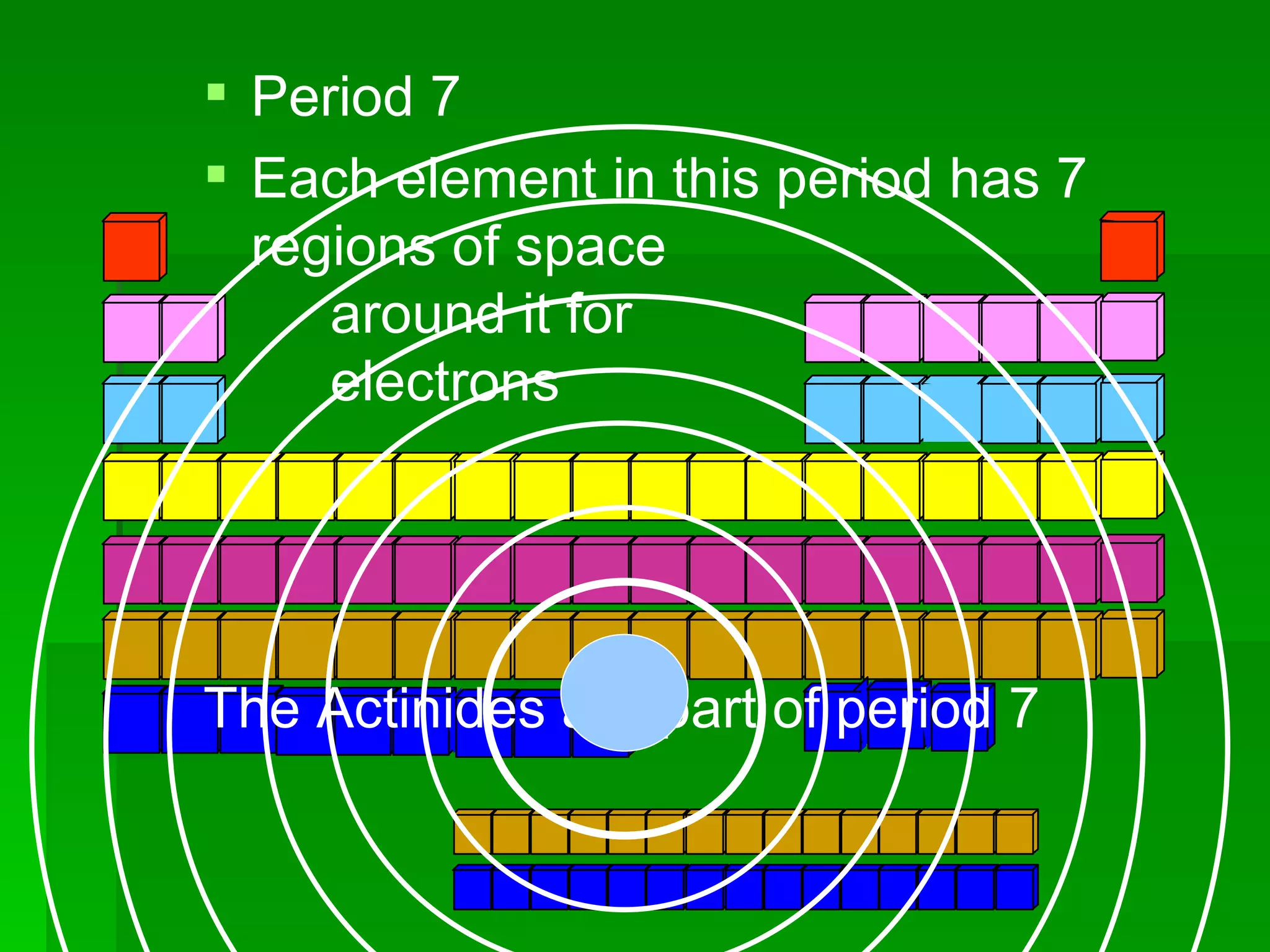
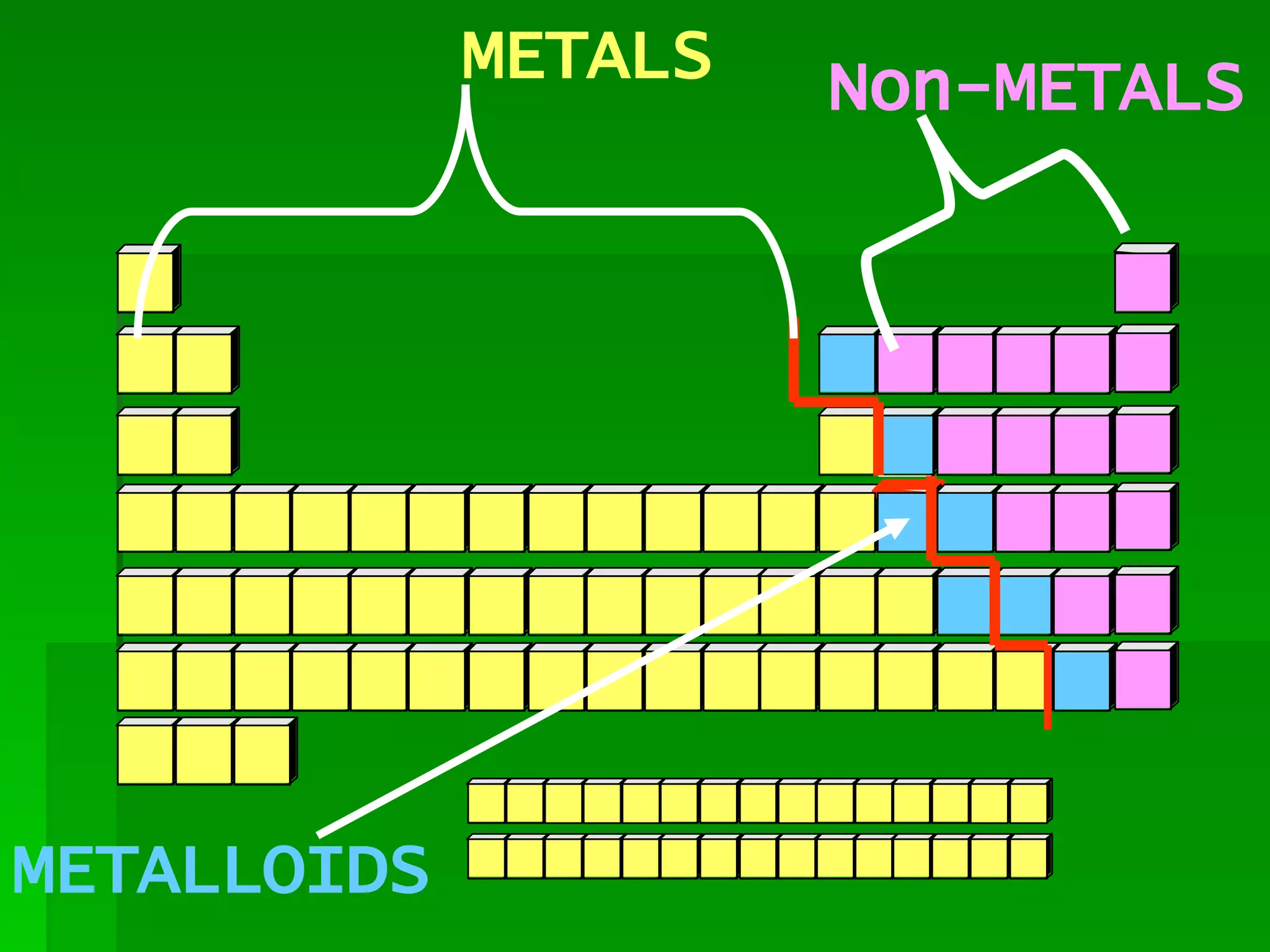
The document summarizes the development of the periodic table of elements from early classifications by Dobereiner and Newlands to Mendeleev's and Meyer's published periodic tables to Moseley's establishment of the ordering by atomic number. It explains key periodic properties including atomic structure and trends in atomic size, and provides an overview of the layout and information contained in the modern periodic table.