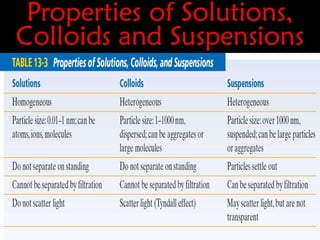
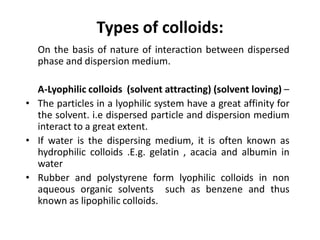
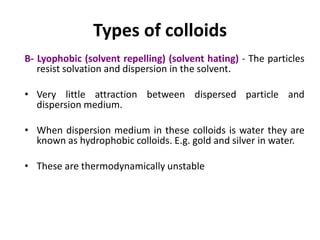
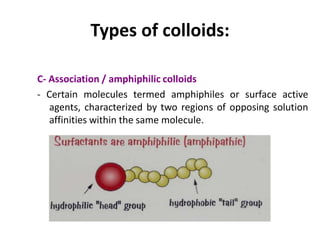
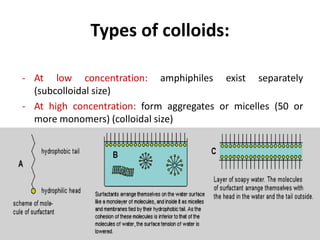
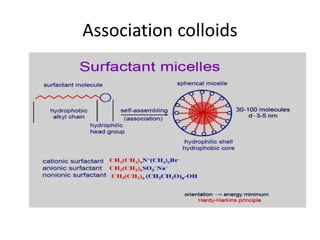
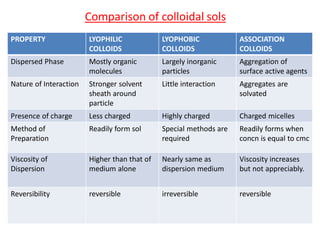
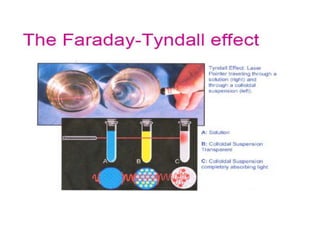
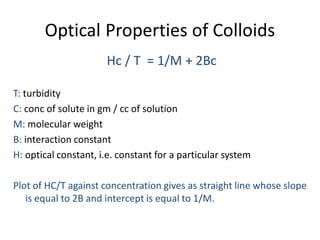
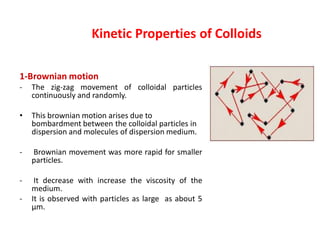
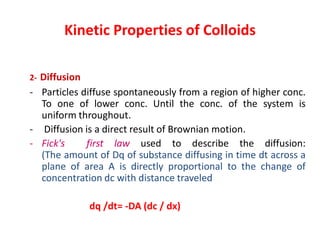
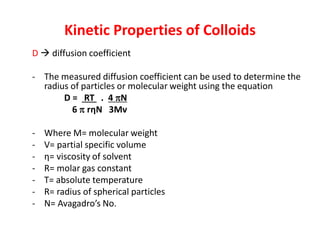
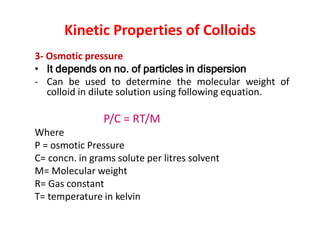
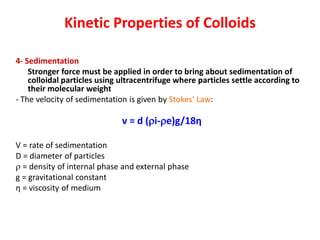
This document discusses colloidal dispersions and their properties. It defines colloidal dispersions as heterogeneous biphasic systems with dispersed particles in the nano size range of 1-1000 nm. Colloids can be classified as lyophilic, lyophobic, or association colloids based on particle-solvent interactions. Key optical properties of colloids include the Tyndall effect, light scattering measurements to determine particle size and molecular weight, and imaging with electron microscopes. Colloids also exhibit kinetic properties like Brownian motion, diffusion, osmotic pressure, and sedimentation rates related to particle size. Electrolytes can cause coagulation or precipitation of colloids according to the Schulze-