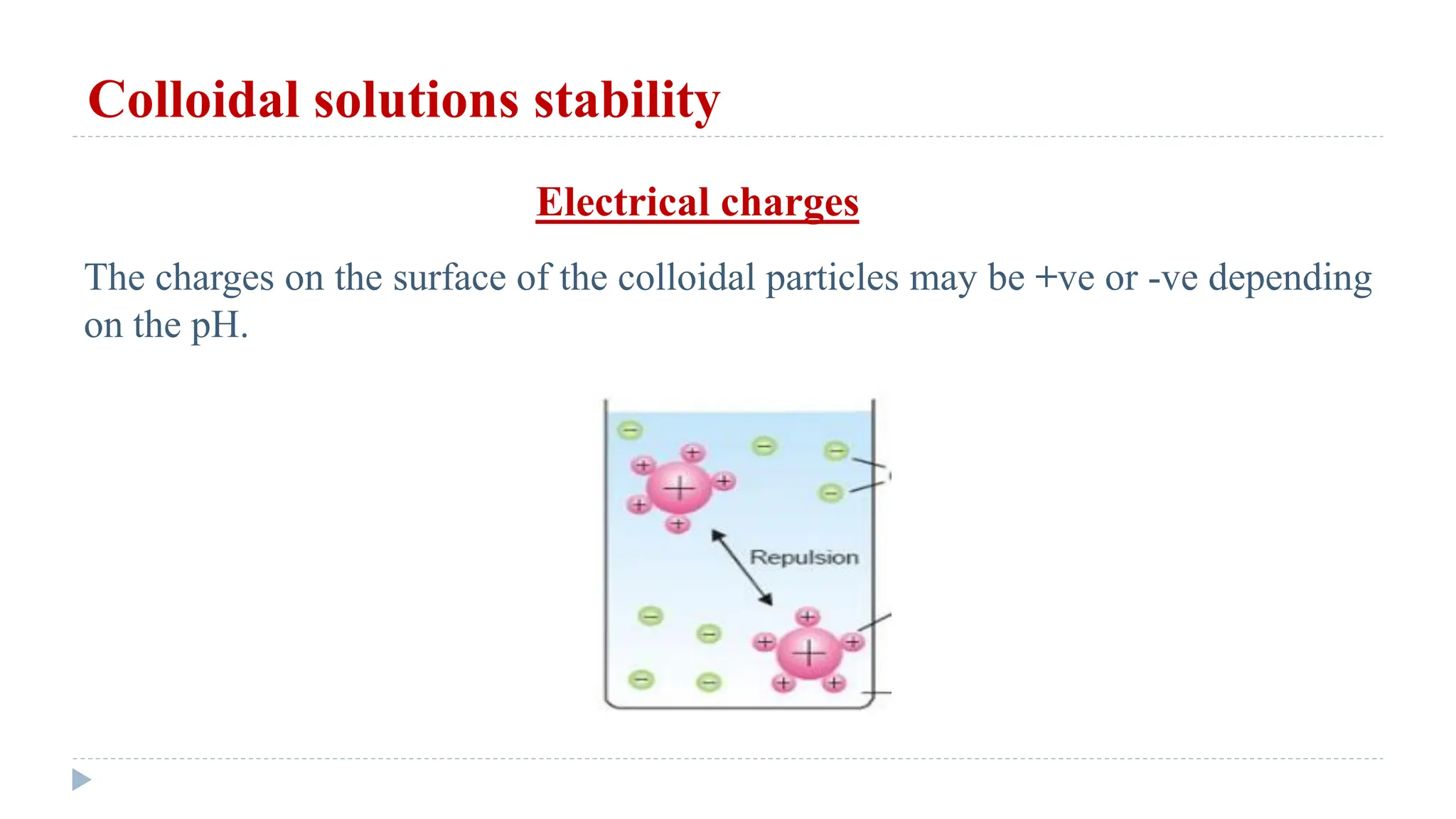
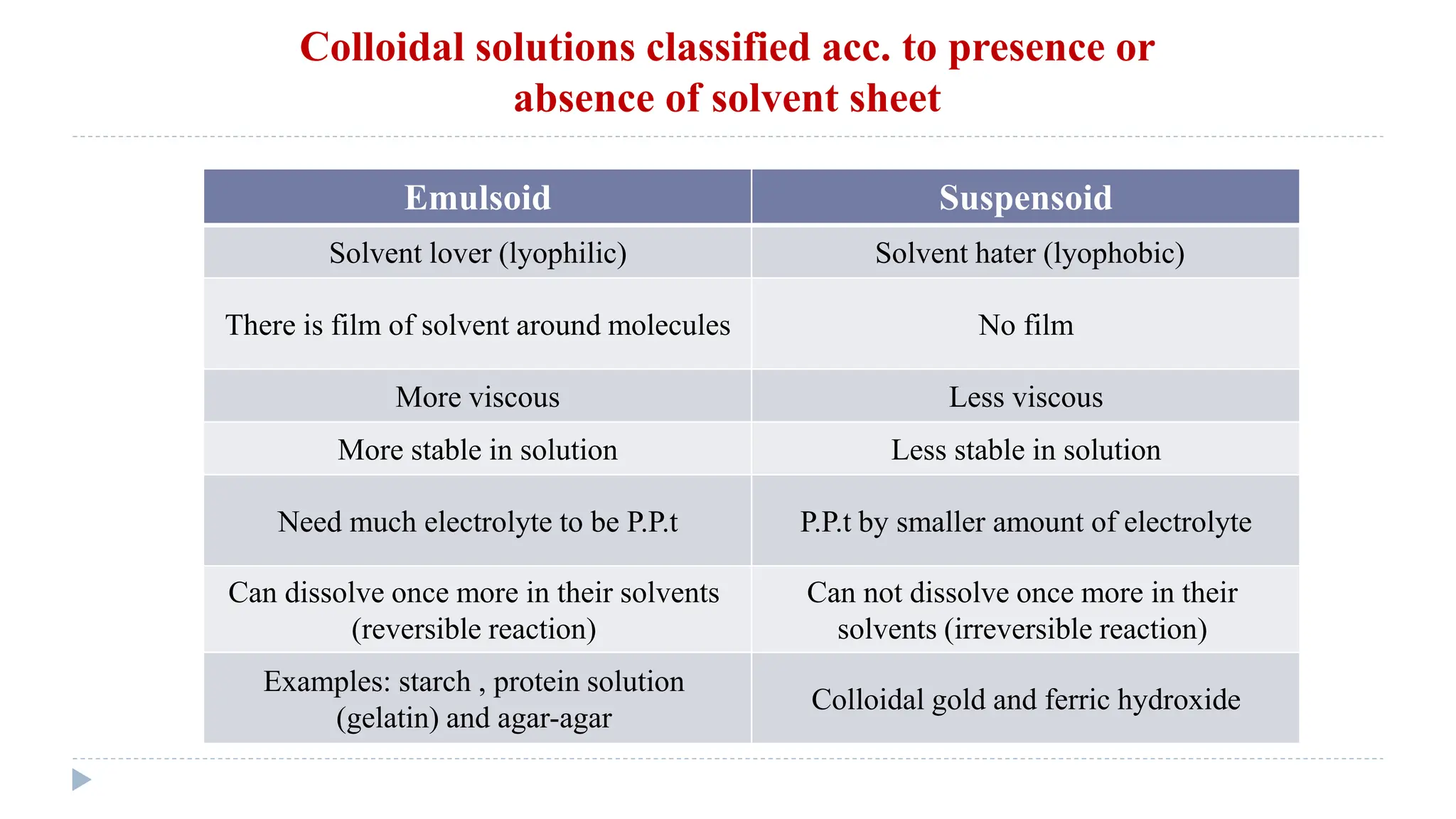
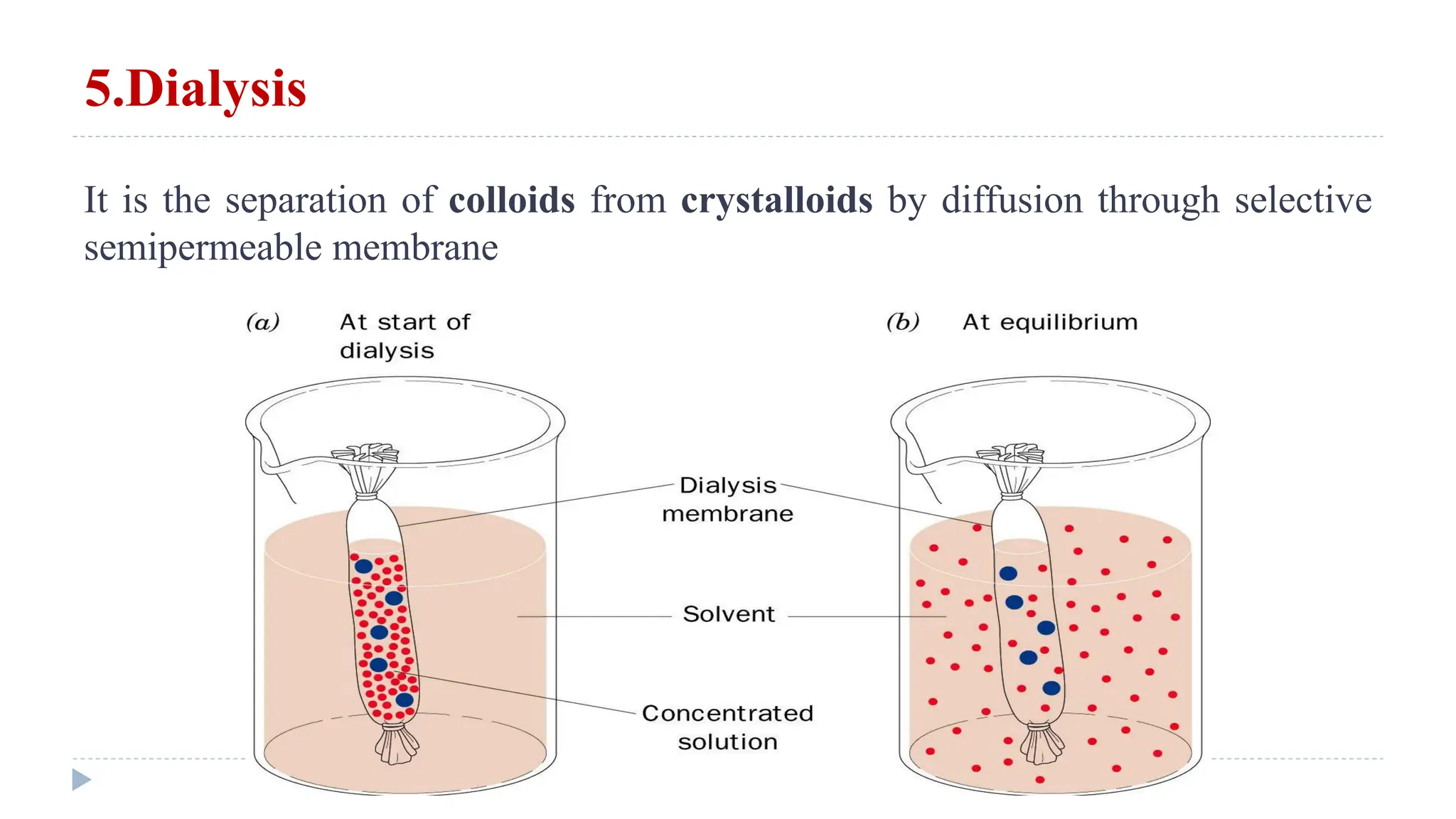
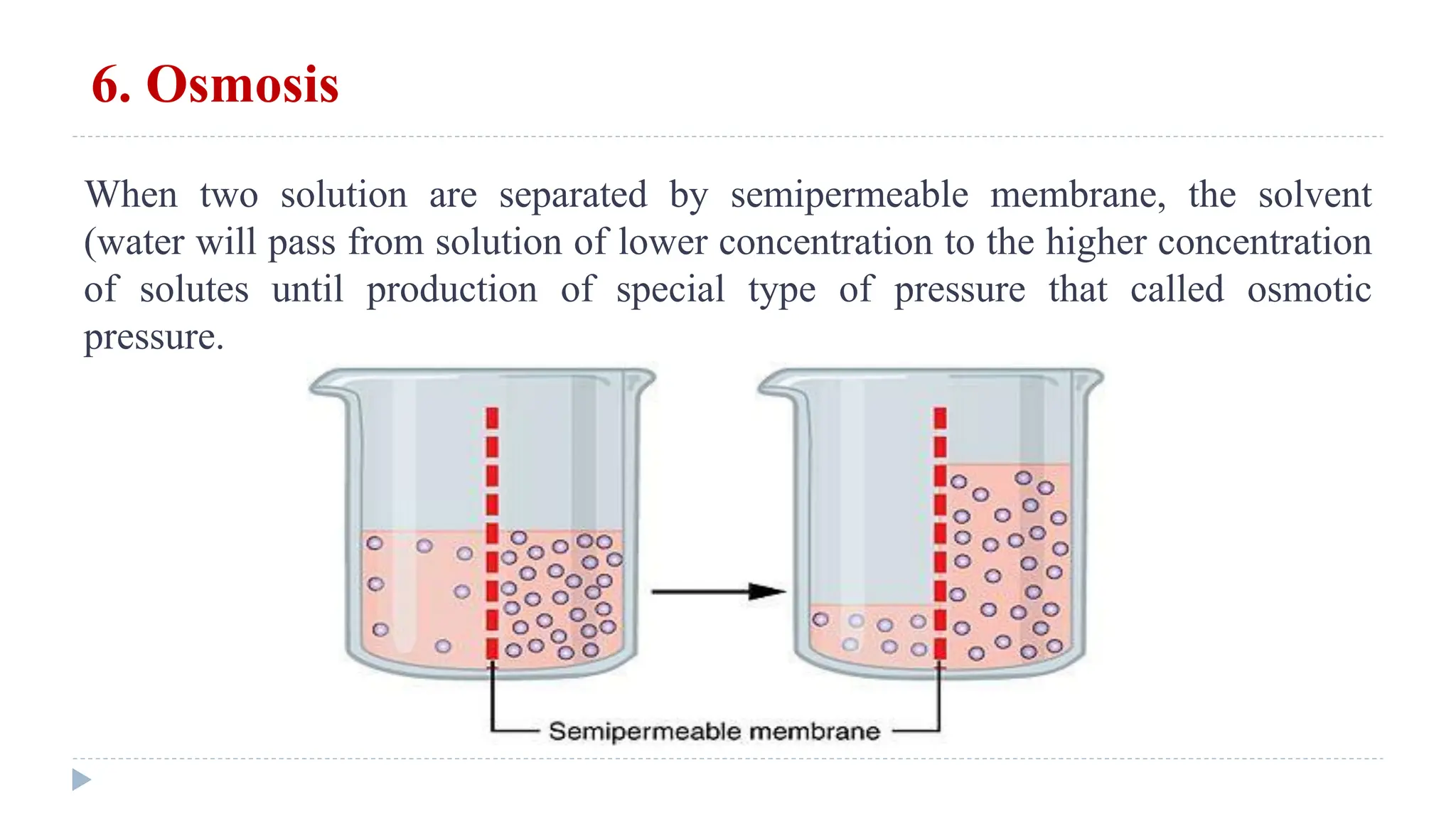
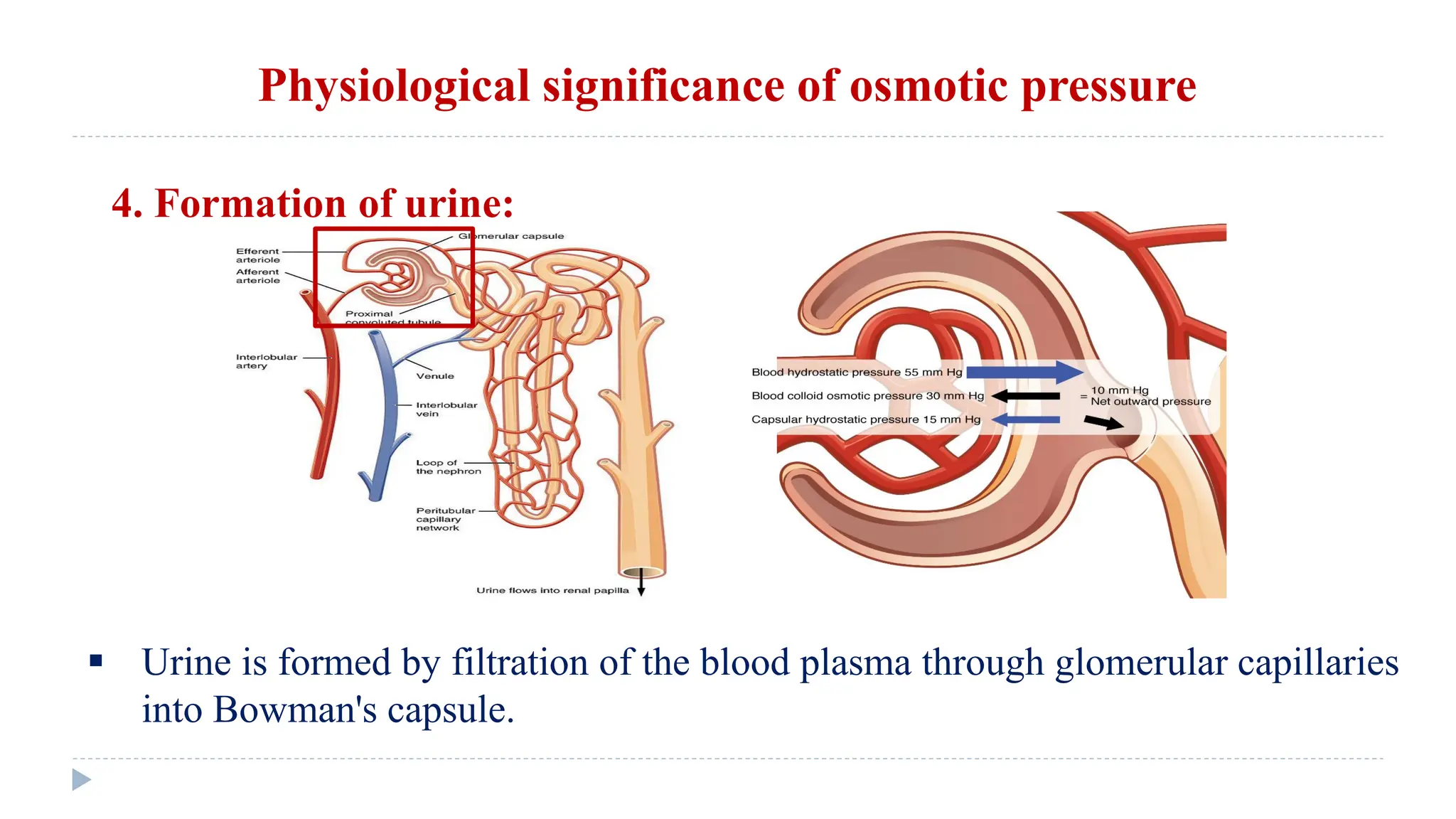
This document defines and classifies solutions, focusing on colloidal solutions. It discusses that solutions are mixtures where one or more substances dissolve in a solvent. Colloidal solutions have particle sizes between 1-200 nm and include sols and gels. They are classified as lyophilic or lyophobic based on whether solvent molecules form a protective layer around particles. The document outlines several properties of solutions, including adsorption, electrical properties, viscosity, surface tension, dialysis, and osmosis. It explains how osmosis governs important physiological processes like formation of urine and regulation of body fluid volumes.