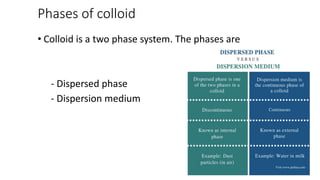
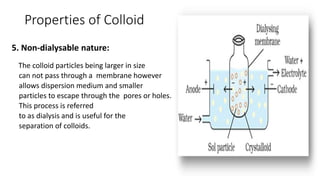
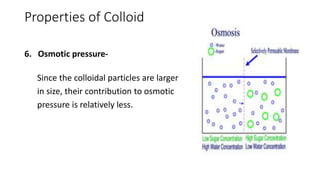
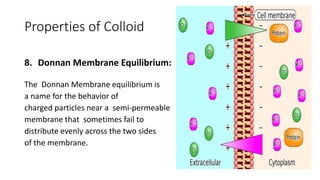
Colloids are heterogeneous mixtures where small insoluble particles are dispersed throughout a liquid medium. Thomas Graham first classified substances as either crystalloids or colloids based on their ability to pass through a membrane. A colloid has particle sizes between 1-100nm, are not visible under a low power microscope but show Brownian motion. Common examples include blood, milk, and fog. Colloids exhibit properties like Tyndall effect, adsorption, precipitation with electrolytes, and non-dialyzability. They play important roles biologically such as in protoplasm, blood coagulation, and fat digestion.