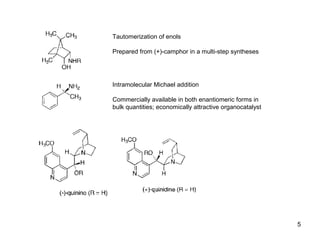
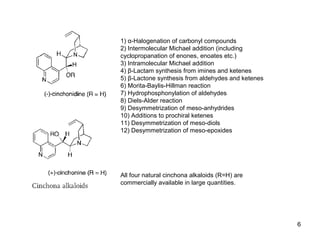
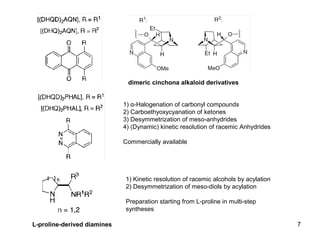
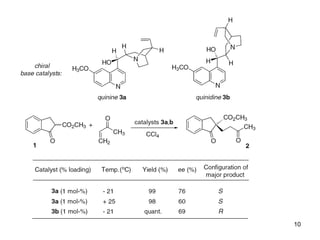
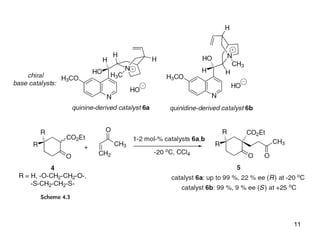
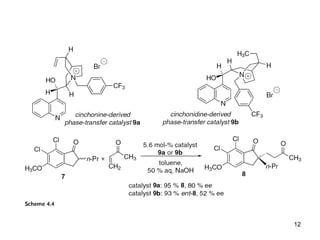
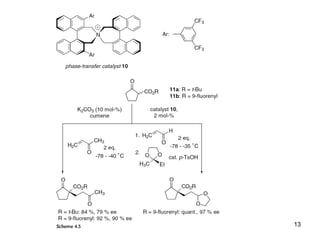
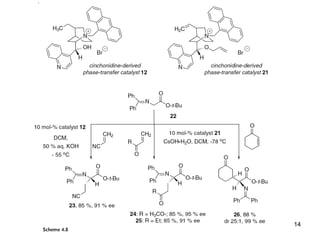
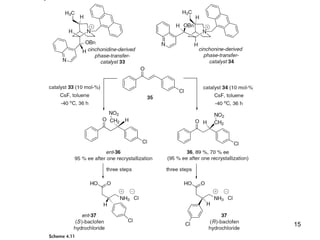
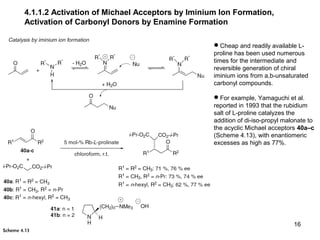
This document provides a tabular survey and overview of various organocatalysts used in asymmetric organocatalysis reactions, including their reaction scopes and commercial availabilities. It discusses how L-proline and other amino acids like L-phenylalanine are commonly used economical organocatalysts that are readily available. It also outlines how cinchona alkaloids are widely used organocatalysts that are commercially available. In general, the document surveys different organocatalysts and their applications in asymmetric reactions.

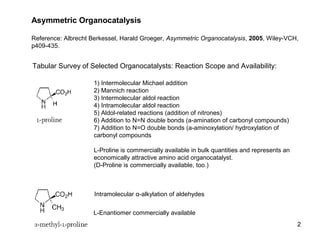
![1) Intermolecular Michael addition
2) Intermolecular aldol reaction
3) [3+2]-Cycloadditions
4) Desymmetrization of meso-diols
5) Desymmetrization of meso-epoxides
Preparation starting from L-proline in
multi-step syntheses
Mannich reaction
Preparation starting from l-proline in
multi-step syntheses
1) Mannich reaction
2) Intermolecular aldol reaction [6.2.1]
Readily accessible, using L-penicillamine
as starting material
3](https://image.slidesharecdn.com/anil-161129171922/85/Organocatalysis-3-320.jpg)
![Intramolecular aldol reaction
Just as L-proline, L-phenylalanine is an
economically attractive amino acid organocatalyst,
readily available in bulk quantities.
1) Intermolecular Michael addition, including alkylation
of heterocyclic aromatics and aniline derivatives
2) [4+2]-Cycloadditions: Diels-Alder reactions
3) [3+2]-Cycloadditions: Nitrone-based reactions
Organocatalysts readily prepared from L-phenylalanine,
methylamine and acetone or piraldehyde
Intermolecular Michael addition
Prepared from L-phenylalanine, methylamine
and glyoxylic acid in a few steps
4](https://image.slidesharecdn.com/anil-161129171922/85/Organocatalysis-4-320.jpg)